
 Background: COVID-19 vaccination is ramping up both in the US as well as throughout the world. Randomized clinical trials looking at the efficacy of the mRNA vaccines demonstrated 94% to 95% reductions in symptomatic cases (Polack 2020). Randomized clinical trials are considered the gold standard when evaluating therapies. However, they are not without limitations. Mass vaccination rollouts do not mirror the settings we see in the highly controlled environments of a randomized clinical trial. It will be important to note if suboptimal adherence to vaccination scheduling and handling logistics will affect the vaccines’ effectiveness. As we continue to vaccinate, we need to further assess the real-world effectiveness of the vaccines.
Background: COVID-19 vaccination is ramping up both in the US as well as throughout the world. Randomized clinical trials looking at the efficacy of the mRNA vaccines demonstrated 94% to 95% reductions in symptomatic cases (Polack 2020). Randomized clinical trials are considered the gold standard when evaluating therapies. However, they are not without limitations. Mass vaccination rollouts do not mirror the settings we see in the highly controlled environments of a randomized clinical trial. It will be important to note if suboptimal adherence to vaccination scheduling and handling logistics will affect the vaccines’ effectiveness. As we continue to vaccinate, we need to further assess the real-world effectiveness of the vaccines.
Article: Dagan N et al. BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. NEJM 2021. PMID: 33626250
Clinical Question: What is the effectiveness of the BNT162b2 mRNA vaccine in a mass vaccination setting?
Population: Data obtained from Clalit Health Services (CHS), the largest of four integrated health care organizations in Israel, which insures 4.7 million patients (53% of the population).
Outcomes:
- Documented infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2),
- Symptomatic Covid-19
- Covid-19–related hospitalization
- Severe illness
- Death
Design: Observational study of all persons who were newly vaccinated during the period from December 20, 2020, to February 1, 2021, were matched to unvaccinated controls in a 1:1 ratio according to demographic and clinical characteristics. Researchers matched vaccine recipients and controls on variables associated with the probability of both vaccination and infection or severity of Covid-19: age, sex, sector (general Jewish, Arab, or ultra-Orthodox Jewish), neighborhood of residence (disease activity and vaccination uptake vary greatly across defined geostatistical areas), history of influenza vaccination during the preceding 5 years, pregnancy, and the total number of coexisting conditions that had been identified by the Centers for Disease Control and Prevention (CDC) as risk factors for severe Covid-19 as of December 20, 2020
Included: Eligibility criteria included age of 16 years or older, not having a previously documented positive SARS-CoV-2 polymerase-chain-reaction (PCR) test and being a member of the health care organization during the previous 12 months.
Excluded: Population groups in which internal variability in the probability of exposure or the outcomes is high and controlling for the high variability is not feasible. Such population groups are persons not having a documented geostatistical living area, those who have had interactions with the health care system during the preceding 3 days that may indicate the start of symptomatic disease and may preclude vaccination, nursing home residents, persons medically confined to the home, or health care workers
Primary Results
- Data from 3,159,136 people
- 1,503,216 (47.6%) vaccinated
- 1,163,534 eligible people
- 596,618 included in the vaccinated cohort
- 1,655,920 (52.4%) not vaccinated
- 596,618 included in the unvaccinated cohort
- Of persons who had 21 or more days of follow-up, 96% received a second dose of vaccine (95% of whom received it before day 24)
- 1,503,216 (47.6%) vaccinated
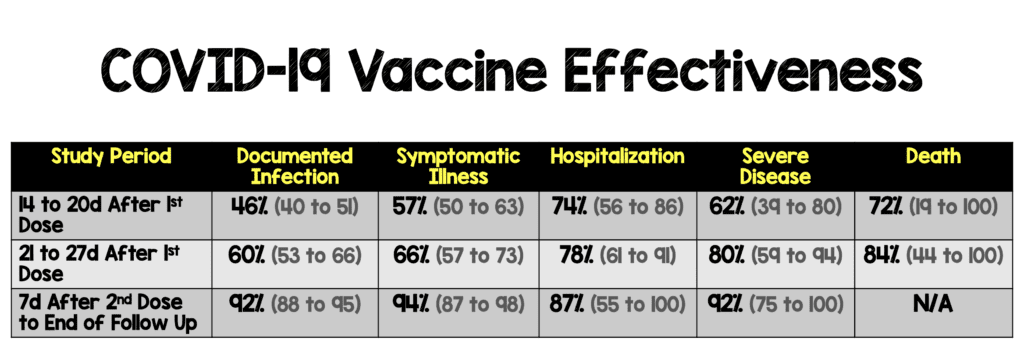
Critical Findings:
Strengths:
- Large population evaluated using real world circumstances
- Large sample size allowed for estimation of vaccine effectiveness for specific subpopulations
- Multiple SARS-CoV-2 variants were present in the population, leading to broader applicability
- Controls and vaccinated patients were well matched in terms of coexisting conditions and known risk factors for severe COVID-19
Limitations:
- The vaccinated and the unvaccinated groups varied in terms of some of the patient risk factor characteristics.
- Confounding variable difference between vaccinated and unvaccinated patients and their health seeking behavior.
- Impossible to take all possible confounding factors into account when matching
- Exclusion of large swaths of the population including healthcare workers and nursing home residents
- B1351 variant of SARS-CoV-2 was rare during the time of this trial and therefore no conclusions can be drawn for effectiveness for this varian
Discussion
- The results of the study are extremely positive for real world effectiveness. This virus has irrevocably altered the course of our history and we finally have a treatment which shows benefit across a wide spectrum of patients.
- The study focuses on the importance of effectiveness versus efficacy in vaccination for COVID. Efficacy is the degree to which a vaccine prevents disease, and possibly also transmission, under ideal and controlled circumstances. Effectiveness meanwhile refers to how well it performs in the real world. Initial data had promising efficacy for the Pfizer covid vaccine and this study shows us that it also has very good effectiveness.
- Symptomatic vs Asymptomatic Infection
- Why is this important?
- RCT data demonstrates marked reductions in symptomatic cases which is critically important
- However, reductions in asymptomatic cases is vital as well. Reductions in asymptomatic cases means that the vaccines can have big impacts on spread of the disease
- A decrease in asymptomatic carriers will lead to decreased spread of the virus and less strain on an already overburdened healthcare system.
- This data is only showing us the effectiveness of the Pfizer covid vaccine and it is important to not extrapolate the data from this study to the effectiveness of other vaccines that are online or coming online soon.
- Multiple variants of the COVID virus were isolated during testing so this study estimates the average effectiveness of the vaccines against various strains.
Authors Conclusions: “This study in a nationwide mass vaccination setting suggests that the BNT162b2 mRNA vaccine is effective for a wide range of COVID-19-related outcomes, a finding consistent with that of the randomized trial”
Our Conclusions: We largely agree with these findings. Vaccinations appear to decrease asymptomatic covid infections, symptomatic infections, hospitalizations and deaths with no significant side effects.
Potential to Impact Current Practice: More vaccines in arms of people will help reduce viral spread and potentially help us get back to a sense of normality while also reducing hospitalizations and death from COVID-19.
Bottom Line: Mass vaccination with the Pfizer vaccine results in a substantial reduction in both asymptomatic and symptomatic COVID-19.
References:
- Polack FP et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. NEJM 2020. PMID: 33301246
- Dagan N et al. BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. NEJM 2021. PMID: 33626250
- Amit S et al. Early Rate Reductions of SARS-CoV-2 Infection and COVID-19 in BNT162b2 Vaccine Recipients. Lancet 2021. PMID: 33610193
Post Peer Reviewed By: Anand Swaminathan, MD (Twitter: @EMSwami) and Salim R. Rezaie, MD (Twitter: @srrezaie)
The post Data from Israel on the BNT162b2 (Pfizer) mRNA COVID-19 Vaccine appeared first on REBEL EM - Emergency Medicine Blog.
