
 Article: Uyeki TM et al. Clinical Practice Guidelines by the Infectious Diseases Society of America: 2018 Update on Diagnosis, Treatment, Chemoprophylaxis, and Institutional Outbreak Management of Seasonal Influenza. Clin Infect Dis 2018. PMID: 30566567
Article: Uyeki TM et al. Clinical Practice Guidelines by the Infectious Diseases Society of America: 2018 Update on Diagnosis, Treatment, Chemoprophylaxis, and Institutional Outbreak Management of Seasonal Influenza. Clin Infect Dis 2018. PMID: 30566567
Background: Influenza is an Emergency Department scourge that we deal with every year. The vast majority of patients recover from uncomplicated influenza without anything more than supportive care but, influenza can cause serious complications. Young children, older adults, pregnant and postpartum women, people with neurologic disorders and patients with certain chronic medical conditions (i.e. COPD, CAD, Diabetes, Immunocompromised states) are at increased risk for these complications. Annual vaccination is the best method to reduce the impact of influenza on morbidity and mortality. Though antiviral medications for influenza are far from perfect, the indications for their use must be understood.
Copious amounts of data pour out each year on immunization, treatment, prophylaxis and diagnosis recommendations making it difficult for emergency clinicians to keep up with the latest. This IDSA guideline, released in December of 2018, is the first clinical practice update in 9 years and summarizes recommendations in each of these areas. The guideline addresses care of children, adults and special populations including pregnant women, postpartum women and immunocompromised patients.


Diagnosis
All recommendations are predicated on only testing if the test results will change management. This is important because if the patient has a high pre-test probability of influenza, the results of the test are unlikely to alter the diagnosis or your planned management.
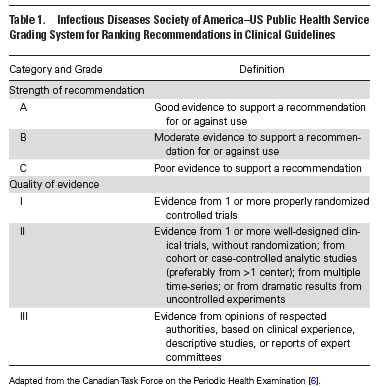
- Nasopharyngeal specimens are preferred for highest yield of tests. (A-II)
- Rapid molecular assays (i.e. nucleic acid amplification tests) are preferred to rapid influenza diagnostic tests. (A-II)
-
Outpatients during times of influenza activity in the community
- Test high-risk patients (i.e. immunocompromised) with influenza-like illness (ILI), pneumonia or nonspecific respiratory illness (A-III)
- Test patients with acute onset respiratory symptoms and either exacerbation of chronic medical conditions (i.e. asthma, COPD) or known influenza complications (A-III)
- Consider testing in non-high risk patients if testing will influence the decision to administer antiviral medications or reduce antibiotic use, further testing or influence chemoprophylaxis decisions (C-III)
-
Hospitalized patients during times of influenza activity in the community
- Test all patients on admission with acute respiratory illness including pneumonia (A-II)
- Test all patients on admission with acute worsening of chronic cardiopulmonary disease (i.e. asthma, COPD, CAD, CHF) (A-III)
- Test all patients on admission who are immunocompromised or high-risk for complications who present with acute respiratory symptoms (even if not classic for influenza) (A-III)
-
Hospitalized patients during times of low influenza activity
- Test all patients requiring hospitalization with acute respiratory illness who have been in contact with a person with influenza (A-II)
- Consider testing in immunocompromised patients or those at high risk for complications if test results will influenza treatment or chemoprophylaxis of contacts
Treatment
Recommended medications: Oral oseltamavir, inhaled zanamivir or intravenous peramivir (A-I)
-
Start antivirals treatment on adults and children with documented or suspected influenza (regardless of immunization history) for the following
- Need for hospitalization for influenza (A-II)
- Outpatients with severe or progressive illness (extensive pneumonia, respiratory failure, hypotension, and fever) regardless of illness duration (A-III)
- Outpatients at high risk of complications of influenza (A-III)
- Children < 2 years old and adults > 65 years (A-III)
- Pregnant women and women < 2 weeks postpartum (A-III)
-
Consider treating adults and children who are not at high risk of complications with documented/suspected influenza for the following:
- Illness onset < 2 days of presentation (C-I) (Oseltamavir utility)
- Symptomatic outpatients with household contacts who are high risk for developing complications of influenza (particularly severe immunocompromise) (C-III)
- Symptomatic healthcare providers who care for patients who are high risk for developing complications (C-III)
-
When should bacterial co-infection be considered?
- Patients with confirmed/suspected influenza with severe disease on presentation (extensive pneumonia, respiratory failure, hypotension) (A-II)
- Patients with initial improvement followed by deterioration (A-III)
- Consider if failure to improve after 3-5 days of antiviral treatment (C-III)
Corticosteroid and immunoglobulin adjunctive therapy is not recommended in any patient group (A-III)

Chemoprophylaxis:
Definition: Administration of antivirals to patients in the absence of influenza-like symptoms.
“Antiviral drugs should not be used for routine or widespread chemoprophylaxis outside of institutional outbreaks.”
Recommended antivirals: oral oseltamivir or inhaled zanamivir)
-
Antiviral pre-exposure chemoprophylaxis can be considered in certain situations
- Adults and children > 3 months at high risk of developing complications from influenza in whom vaccination is contraindicated, unavailable or, expected to have poor effectiveness (C-II)
- Adults and children > 3 months at highest risk of complications (recipients of hematopoietic stem cell transplant, lung transplant) for duration of influenza season (B-II)
- Short-term chemoprophylaxis in unvaccinated adults and children > 3 months at high-risk (along with inactivated influenza vaccine) (C-II)
-
Antiviral post-exposure chemoprophylaxis can be considered in certain situations:
- Consider in asymptomatic adults and children > 3 months at very high risk of developing complications and in whom vaccination is contraindicated, unavailable or expected to have low effectiveness (C-II)
- Consider for adults and children > 3 months (along with influenza vaccination) who are unvaccinated and are household contacts of people at very high risk of complications (C-II)
For More on This Topic Checkout:
- REBEL EM: The Tamiflu Debacle
- ALiEM: Neuraminidase Inhibitors for Influenza – The Truth, the Whole Truth and Nothing But the Truth, Finally
Post Peer Reviewed By: Salim R. Rezaie, MD (Twitter: @srrezaie)
The post IDSA Guideline on Seasonal Influenza Management 2018 appeared first on REBEL EM - Emergency Medicine Blog.
