
 Background: The combination of vitamin C, hydrocortisone and thiamine in sepsis has been a topic of hot debate in the past couple years. There is a hypothetical pathophysiological basis to make an argument for the use of this combination of medications, but as with anything it is important to ensure there are no untoward effects either. In Dr. Marik’s before and after study [1] we saw some pretty amazing results showing that treatment reduced hospital mortality by 31.9% (Treatment Group 8.5% vs Control Group 40.4%). Too good to be true? Well in short, YES…the major issues with this study were it was not a randomized controlled trial, had a small sample size, was a single center study, and had significant selection bias. Well we finally have our first randomized controlled trial evaluating the “metabolic cocktail” in a general population of septic shock adult patients.
Background: The combination of vitamin C, hydrocortisone and thiamine in sepsis has been a topic of hot debate in the past couple years. There is a hypothetical pathophysiological basis to make an argument for the use of this combination of medications, but as with anything it is important to ensure there are no untoward effects either. In Dr. Marik’s before and after study [1] we saw some pretty amazing results showing that treatment reduced hospital mortality by 31.9% (Treatment Group 8.5% vs Control Group 40.4%). Too good to be true? Well in short, YES…the major issues with this study were it was not a randomized controlled trial, had a small sample size, was a single center study, and had significant selection bias. Well we finally have our first randomized controlled trial evaluating the “metabolic cocktail” in a general population of septic shock adult patients.
REBEL Episode 74 – Is it all About the VITAMINS in Sepsis?
Click here for Direct Download of Podcast
From Research to Practice Episode 1.0 – The VITAMINS Trial
[embedyt] https://www.youtube.com/watch?v=gCqO_TjNfT0[/embedyt]
Also Be Sure to Checkout our YouTube Channel

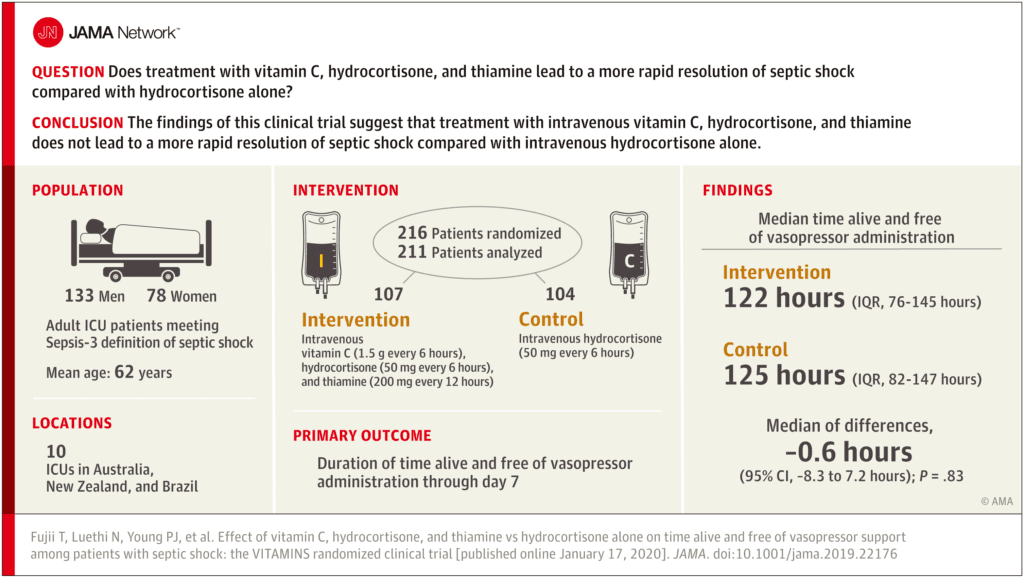
Clinical Question:
- Does treatment with vitamin C, hydrocortisone, and thiamine lead to a more rapid resolution of septic shock compared with hydrocortisone alone?
What They Did:
- The VitamIn C HydrocorTisone and ThiAMINe in patients with Septic shock Trial (VITAMINS Trial)
- Prospective, Feasibility, Pilot, Multicenter, Randomized, Open-Label, Parallel Group Controlled Trial
- Patients recruited from 10 ICUs in Australia, New Zealand, and Brazil
- Treatment:
- Vitamin C (Ascorbic Acid) 1.5g q6hr IV (while in the ICU, until shock resolution for a max of 10 days) PLUS
- Thiamine 200mg q12hrs IV (while in the ICU, until shock resolution for a maximum of 10 days) PLUS
- Hydrocortisone 50mg q6hrs IV (while in the ICU, until shock resolution or for a max of 7 days, then tapered or stopped)
- Control:
- Hydrocortisone 50mg q6hrs IV (while in the ICU, until shock resolution or for a max of 7 days, then tapered or stopped)
Outcomes:
-
Primary: Time alive and free of vasopressors at day 7 (168hrs) after randomization
- Definition = patient being alive at discontinuation of all vasopressors for at least 4 hours in the presence of a MAP >65mmHg for the same 4-hour period as recorded in the ICU charts and censored at 7d
-
Secondary:
- 28d mortality
- 90d mortality
- ICU mortality
- Hospital mortality
- 28d cumulative vasopressor-free days
- 28 cumulative mechanical ventilation-free days
- 28 renal replacement therapy-free days
- Change in SOFA score at day 3
- 28d ICU free days
- Hospital length of stay
Inclusion:
- ≥18 years of age
- Septic shock patients (based on the Sepsis-3 definition) in the ICU
- Lactate >2mmol/L, despite adequate fluid resuscitation
- Need for continuous vasopressor therapy to keep mean arterial pressure (MAP) >65mmHg for >2hrs
Exclusion:
- Age <18 years
- DNR order
- Imminent death
- Onset of septic shock >24hrs
- Contraindication to any study drug
- Use of study drugs for other reasons
- Pregnancy
- Known HIV infection
- Known glocse-6 phosphate dehydrogenase (G6PD) deficiency
- Transferred from another ICU or hospital
- Known oxalate nephropathy or hyperoxaluria, short bowel syndrome, severe fat-malabsorption, acute beri-beri disease, acute Wernicke’s encephalopathy, malaria, scurvy, Addison’s disease, Cushing’s disease
- Receiving treatment for systemic fungal infection or documented Stronglyloides infection
- Known chronic iron overload due to iron storage diseases
Results:
- 786 patients were assessed for eligibility
- 570 were excluded based on preset criteria (most common reason was diagnosis of sepsis shock >24hrs)
- 216 patients randomized
- 211 patients included in the primary analysis (5 patients withdrew consent or consent was not obtained)
- The primary sites of infection were pulmonary (≈30%), GI (≈30%), and urinary (≈15%)
- Treatment in both groups was given on average for a mean of 3.4d
-
Median Time Alive and Vasopressor Free up to Day 7 (Primary Outcome):
- Treatment: 122.1hrs (Range 76.3 – 145.5hrs)
- Control: 124.6hrs (Range 82.1 – 147.0hrs)
- 95% CI -8.3 to 7.2hrs
- P = 0.83
- No statistically significant difference
- Median Change in SOFA score at Day 3:
- Treatment: -2 (-4 to 0)
- Control: -1 (-3 to 0)
- 95%CI 01.9 to 0.1
- P = 0.02
- No difference in 28d, 90d, ICU mortality, or hospital mortality
- No difference in 28d vasopressor free days, mechanical ventilation free days, renal replacement therapy free days, or ICU free days
- No difference in vasopressor dose during first 10 days
- No difference in major adverse events reported (2 in the intervention arm and 1 in the control arm)
Strengths:
- First randomized clinical trial evaluating the use of Vitamin C, thiamine, and hydrocortisone in a general population of septic shock patients using the same cocktail protocol as the Marik study [1]
- Although, moderate in size, this trial is much larger than the Marik study [1] and the CITRIS-ALI trial [7]
- Trial protocol with predefined statistical analysis plan was published before the study began recruitment
- Randomization was properly performed and was concealed
- Updated sample size calculations based on pooled standard deviations for first 60 patients as there was an absence of accurate and/or current data to estimate the sample size. This was inflated by 15% to account for consent withdrawal and increase statistical power. The robustness of the sample size estimation was further confirmed after recruitment of 108 patients
- Post-hoc analysis of the duration of vasopressor use was assessed censoring patients who died before resolution of shock at the time of death to avoid survivor bias
- Provides an intervention for up to 10 days which gives a sufficient treatment period for the treatment to have any potential effect (The Marik before and after trial [1] provided treatment for up to 4 days)
- Very few patients were lost to follow up, minimizing attrition bias
- Study was performed in both high- and middle-income countries helping to improve external validity of the results
Limitations:
- Open label study, meaning all site personnel were aware of study interventions assigned to participants potentially creating a performance and ascertainment bias
- The average time from meeting eligibility criteria to the first dose of medications:
- Vitamin C: ≈12hrs – Range 5.7 – 19.0hrs
- Hydrocortisone: ≈8.9hrs – Range 4.0 – 15.0hrs
- Add to this, time from ICU admission to randomization: ≈12hrs
- This is >24hrs before medication given. These medications may have been given too late to have a clinical effect
- Time to the administration of antibiotics was not collected and the appropriateness of antibiotics was not collected. The current pillars of sepsis management include early identification, source control, and early appropriate antibiotics.
- The amount of fluids received was not documented in this trial.
- Thiamine administration was allowed in the control group at the discretion of the attending ICU clinicians. Thiamine levels were not measured in this trial making it unclear if patients had thiamine deficiency at randomization or if this deficiency was corrected. This was only 7.7% of the control arm and most likely not clinically significant
- Intervention group had lower APACHE III scores (77.4 vs 83.3), higher lactate levels (4.2 vs 3.3), higher WBC (17.5 vs 15.3), and more likely to have received milrinone (5.6% vs 1.9%)
- Study was not powered to detect any differences in secondary outcomes (i.e. mortality or other patient oriented outcomes). The statistically significant difference in SOFA score at day 3 should therefore be cautiously interpreted
- Individual effects of vitamin C and thiamine were not assessed separately due to the study design
- Adverse events were only reported when adjudicated. Patients were not examined for all possible adverse effects (i.e. oxaluria) that could potentially develop
Discussion:
- There have been lots of exciting clinical trials for critically ill septic patients over the years (i.e. early goal directed therapy, tight glycemic control, activated protein C, etc…). Many of these trials generated overall excitement when first published, but failed in replication during external validation. Recall the publications of ProCESS [3], ARISE [4], and ProMISe [5] debunking early goal directed therapy as standard care in sepsis. Credit was given to Manny Rivers, as he forever changed how we managed patients with sepsis with his initial publication in 1999 [2]. He was not ridiculed for being wrong, but instead…he put sepsis on the map. In this light, Paul Marik should be applauded for continuing to further our understanding of sepsis management. Although this trial is a negative one, without research we cannot continue to improve the way we care for patients.
- Patients in this study received lower doses of vitamin C compared to the CITRIS-ALI trial [7] (1.5g q6hrs for up to 10days vs 50mg/kg actual body weight q6hrs for 96hrs). However, plasma concentrations of vitamin C in both trials were similar at 6hrs. There was also a 28 all-cause mortality difference in CITRIS-ALI (Vit C 29.8% vs Placebo 48.3%) compared to VITAMINS (Treatment 22.6% vs Control 20.4%). I am not suggesting causality that vitamin C improves outcomes, but that it is unclear what dose of vitamin C we should use if at all
- Unblinding in this trial is a huge issue. It is unclear which direction clinician biases would run here – they may be pro-cocktail biasing study towards intervention or, they may be skeptics like us biasing towards the control group.
- Currently the VICTAS Trial, another RCT of vitamin C is recruiting from 43 sites and has 500 patients.
- In an accompanying editorial [8] by Andre Kalil, MD he eloquently states:
“Moreover, use of high-dose vitamin C in combination or alone “just in case” or as a “measure of last re- sort,” aside from providing no survival benefits, could have several other potential consequences, including diverting funding from needed research to examine sepsis mechanisms and diagnostics; stifling the development of other sepsis therapies; perpetuating false hopes for patients, families, and clinicians; and delaying proven lifesaving therapies such as prompt initiation of antibiotic therapy.”
Author Conclusion: “In patients with septic shock, treatment with intravenous vitamin C, hydrocortisone, and thiamine, compared with intravenous hydrocortisone alone, did not significantly improve the duration of time alive and free of vasopressor administration over 7 days. The finding suggests that treatment with intravenous vitamin C, hydrocortisone, and thiamine does not lead to a more rapid resolution of septic shock compared with intravenous hydrocortisone alone.”
Clinical Take Home Point: In this trial, treatment of septic shock with intravenous vitamin C, hydrocortisone, and thiamine did not improve duration of time alive and free of vasopressor administration over 7days compared to IV hydrocortisone alone. Believers will say, an average time of >24hrs from ICU admission to first dose of medication set this trial up for failure, but the bigger question is at this point should we use the “metabolic cocktail” in sepsis or not? I am in the camp that current evidence does not support its use. We will just have to wait and see how the subsequent trials shake out.
References:
- Marik PE et al. Hydrocortisone, Vitamin C and Thiamine for the Treatment of Severe Sepsis and Septic Shock: A Retrospective Before-After Study. Chest 2016. PMID: 27940189
- Rivers E et al. Early Goal-Directed Therapy in the Treatment of Severe Sepsis and Septic Shock. NEJM 2001. PMID: 11794169
- ProCESS Investigators. A Randomized Trial of Protocol-Based Care for Early Septic Shock. NEJM 2014. PMID: 24635773
- ARISE Investigators. Goal-Directed Resuscitation for Patients with Early Septic Shock. NEJM 2014. PMID: 25272316
- Mouncey PR et al. Trial of Early, goal-Directed Resuscitation for Septic Shock. NEJM 2015. PMID: 25776532
- Fujii T et al. Effect of Vitamin C, Hydrocortisone, and Thiamine vs Hydrocortisone Alone on Time Alive and Free of Vasopressor Support Among Patients With Septic Shock: The VITAMINS Randomized Clinical Trial. JAMA 2020. [Epub Ahead of Print]
- Fowler AA 3rd et al. Effect of Vitamin C Infusion on Organ Failure and Biomarkers of Inflammation and Vascular Injury in Patients with Sepis and Severe Acute Respiratory Failure: The CITRIS-ALI Randomized Clinical Trial. JAMA 2019. PMID: 31573637
- Kalil AC. Lack of Benefit of High-Dose Vitamin C, Thiamine, and Hydrocortisone Combination for Patients With Sepsis. JAMA 2020. [Epub Ahead of Print]
For More Thoughts on This Topic Checkout:
- FOAMCast: VITAMINS Trials – Vitamin C + Hydrocortisone + Thiamine in Septic Shock
- Critical Care Reviews: CCR17 Cure for Sepsis
- REBEL EM: The Marik Protocol – Have We Found a “Cure” for Severe Sepsis and Septic Shock?
- REBEL EM: CITRIS-ALI – Vitamin C in Patients with Sepsis and Severe Acute Respiratory Failure
- Critical Care Reviews: CCR20 VITAMINS Trial Results Session
- EM Lit of Note: Happy VITAMINS Day!
- PulmCrit: Metabolic Resuscitation – Was the Answer Inside us all Along?
- The Bottom Line: VITAMINS
- EMNerd: The Case of the Sour Remedy Continues
- JournalFeed: VITAMINS for Septic Shock? Hydrocortisone, Ascorbic Acid, and Thiamine for Septic Shock
- First10EM: Sepsis is Scurvy?? Vitamin C, Thiamine, and Steroids
Post Peer Reviewed By: Anand Swaminathan, MD (Twitter: @EMSwami)
Support the Show by Paying & Claiming 1.0hrs of CME/CEH by Clicking on the Logo Below
Subscribe to the CME Collective for more CME (Use the Code: REBEL for 10% Discount)
The post REBEL Cast Ep74 – Is it all About the VITAMINS in Sepsis? appeared first on REBEL EM - Emergency Medicine Blog.


