
 Background: Science by press release. Not the way any of us would choose to operate but, the COVID pandemic has made this a reality. It’s vital that we understand that while pharmaceutical companies have a responsibility to release this information, we as clinicians should not be practicing medicine based on press releases. Of course, these press releases don’t only originate from pharma. On January 22nd, 2021, the Montreal Heart Institute released a statement about the results from the COLCORONA study investigating the use of colchicine in COVID-19. The press release painted a very positive picture but, does the pre-peer reviewed publication stand up?
Background: Science by press release. Not the way any of us would choose to operate but, the COVID pandemic has made this a reality. It’s vital that we understand that while pharmaceutical companies have a responsibility to release this information, we as clinicians should not be practicing medicine based on press releases. Of course, these press releases don’t only originate from pharma. On January 22nd, 2021, the Montreal Heart Institute released a statement about the results from the COLCORONA study investigating the use of colchicine in COVID-19. The press release painted a very positive picture but, does the pre-peer reviewed publication stand up?
REBEL Cast Ep95: Colchicine in COVID (COLCORONA)? Don’t Believe the Hype.
Paper: Tardif JC et al. Efficacy of Colchicine in Non-Hospitalized Patients with COVID-19. MedRxiv 2021[Link is HERE] ClinicalTrials.gov
Clinical Question: Does colchicine reduce the risk of hospitalization or death in outpatients with COVID-19 who are at high-risk for decompensation?
Population: Patients ≥ 40 years of age with a diagnosis of COVID-19 who could be enrolled within 24 hours of diagnosis, were not currently hospitalized and not under immediate consideration for hospitalization. All patients had to have at least one of the following high-risk criteria:
- Age > 70
- Obesity (BMI > 30 kg/m2)
- Diabetes
- Uncontrolled hypertension (SBP > 150 mm Hg)
- Known respiratory disease
- Known heart failure
- Known coronary artery disease
- Fever > 38.4 C within the last 48 hours
- Dyspnea at the time of presentation
- Bicytopenia
- Pancytopenia
- Combination of high neutrophil and low lymphocyte count
Intervention: Colchicine 0.5 mg Q12 X 3 days followed by 0.5 mg Q24 X 27 days
Control: Placebo
Outcome (Primary): Composite endpoint of death or hospitalization due to COVID-19 within 30 days following randomization (intention to treat analysis)
Outcomes (Secondary): Components of the composite primary endpoint (death or hospitalization within 30 days), mechanical ventilation within 30 days of randomization
Design: Randomized, double-blind, placebo-controlled, investigator-initiated trial. Interim analyses pre-specified at 25, 50 and 75% enrollment
Exclusion:
- Pregnant women
- Women not practicing adequate contraception
- Inflammatory bowel disease
- Chronic diarrhea
- Pre-existing malabsorption
- Pre-existing neuromuscular disease
- GFR < 30 ml/min
- Severe liver disease
- Current treatment with colchicine
- Current chemotherapy
- History of colchicine sensitivity
Results:
Primary Results:
- Enrollment from March 2020 to December 2020
- 4488 patients underwent randomization
- Primary endpoint status known for 97.9% of patients
- 4159 patients found to be SARS-CoV2 PCR positive
- Patients enrolled a mean of 5.3 days after symptom onset
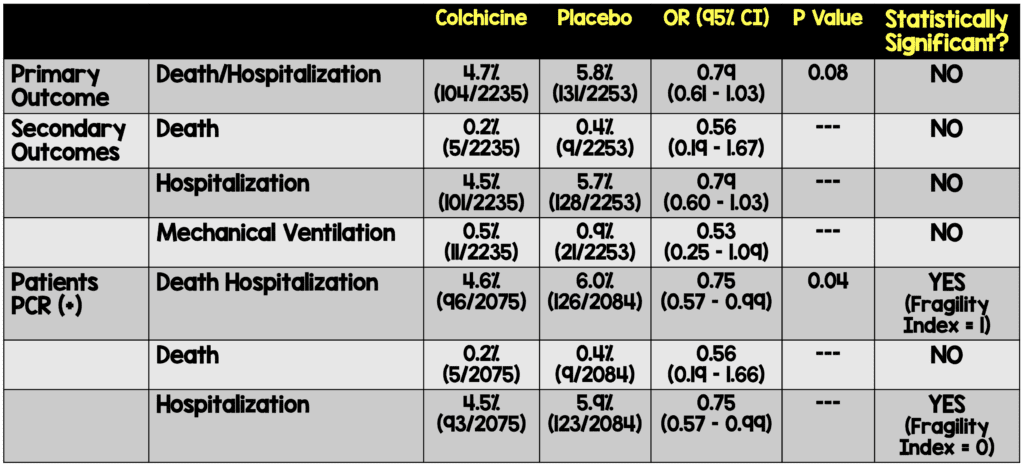
Critical Results:
Adverse Events:
- Diarrhea:
- Colchicine: 300/2195 (13.7%)
- Placebo: 161/2217 (7.3%)
- P<0.0001
Strengths:
- Randomized, double-blind, placebo-controlled trial
- Multicenter, multinational study increasing external validity
- Baseline characteristics that were reported are well-balanced
- Evaluated patients in the outpatient setting, early in disease with a medication that is orally administered and inexpensive
Limitations:
- Primary endpoint was composite of outcomes that are not equivalent (death and hospitalization are not the same)
- Enrollment was non-consecutive which may introduce bias
- Study powered to find a 25% relative risk reduction which may have been overly ambitious
- Study stopped at 75% enrollment
- Follow up was only 30 days
Discussion:
- Regardless of press release and spin of data, this was a negative study. It is only after manipulation of the data that the authors were able to show a small benefit in preventing hospitalization.
- Secondary analysis removed patients who were PCR negative
- The authors state that this was a prespecified analysis but, there is no indication of this on clinicaltrials.gov
- PCR results are not 100% sensitive. Exclusion of patients who were PCR negative may have resulted in exclusion from secondary analysis of patients who actually did have COVID
- Statistical significance was driven by hospitalization rate (subjective) not mortality (objective)
- Regardless, this is a post-hoc analysis and should not be used to change management
- Colchicine has considerable toxicity including multisystem organ dysfunction
Author Conclusion: “Among non-hospitalized patients with COVID-19, colchicine reduces the composite rate of death or hospitalization. “
Our Conclusions: The authors have taken substantial liberties in their interpretation. Based on the sound methodology set up for this study, there is no significant clinical benefit for colchicine in the treatment of COVID19.
Bottom Line: Colchicine should not be given to patients with COVID19 outside of the setting of a clinical study
ADDENDUM 03/05/2021 – RECOVERY Trial Press Release
RECOVERY Trial: Hospitalized Pts w/ #COVID19
28d Mortality
-20% colchicine
-19% usual care alone
-RR 1.02; 95% CI 0.94-1.11; p=0.63
-Outpts not included
-Await full publicationPress Release: https://t.co/uqzCBDRIjc@stemlyns: https://t.co/dz1HkP2TNu#FOAMed #COVID19FOAM pic.twitter.com/Rxcm7wDdiC
— Salim R. Rezaie, MD (@srrezaie) March 5, 2021
References:
- Tardif JC et al. Efficacy of Colchicine in Non-Hospitalized Patients with COVID-19. MedRxiv 2021[Link is HERE] ClinicalTrials.gov
For More Thoughts on This Topic Checkout:
- ALiEM:
- FOAMCast:
Post Peer Reviewed By: Salim R. Rezaie, MD (Twitter: @srrezaie)
The post REBEL Cast Ep95: Colchicine in COVID (COLCORONA)? Don’t Believe the Hype. appeared first on REBEL EM - Emergency Medicine Blog.
