
 Background: Injuries are a major cause of death worldwide. Hemorrhage accounts for about 1/3 of all trauma deaths and as such, it should be our goal to find treatments to decrease death from hemorrhage. Our bodies have a finely tuned system that allows blood to flow freely and not clot too easily while also allowing the body to form clots when needed. This balance is upset in trauma by loss of blood and factors, acidosis, hypothermia and the inflammatory cascade.
Background: Injuries are a major cause of death worldwide. Hemorrhage accounts for about 1/3 of all trauma deaths and as such, it should be our goal to find treatments to decrease death from hemorrhage. Our bodies have a finely tuned system that allows blood to flow freely and not clot too easily while also allowing the body to form clots when needed. This balance is upset in trauma by loss of blood and factors, acidosis, hypothermia and the inflammatory cascade.
Tranexamic Acid (TXA) is a synthetic derivative of lysine that inhibits fibrinolysis and aids in stabilizing clots.The CRASH-2 trial demonstrated a 1.5% absolute decrease in death in trauma patients with significant bleeding randomized TXA (NNT = 66). The result of this massive, multinational study was that TXA became standard care in many trauma systems around the world. Despite this, there remained skeptics. One major criticism was that the study was performed in under-resourced systems and may not be generalizable to care in advanced trauma systems (those with regionwide systems of trauma care that facilitate rapid access to life-saving critical are). The PATCH-trauma researchers sought to address this critique.
Article: PATCH-Trauma Investigators and ANZICS Clinical Trial Group. Prehospital Tranexamic Acid for Severe Trauma. NEJM 2023. PMID: 37314244
Clinical Question: In advanced trauma systems, does prehospital administration of TXA increase the rate of survival with a favorable neurologic outcome in patients at risk for trauma-induced coagulopathy?
Population: Adults (> 18 years of age) with suspected severe traumatic injuries at risk for trauma-induced coagulopathy (assessed using the Coagulopathy of Severe Trauma (COAST) score). These were patients who were treated by paramedics or physicians and transported to a participating trauma center and could be administered TXA or placebo within 3 hours of injury.
Coagulopathy of Severe Trauma (COAST Score)
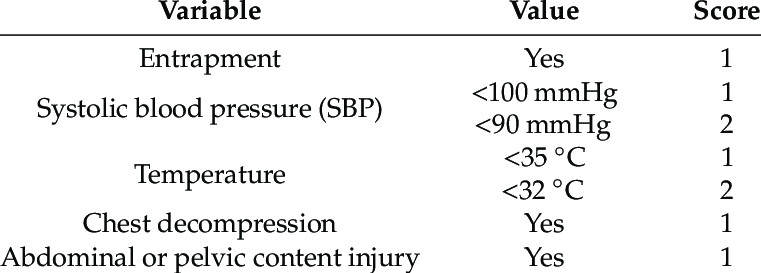
Outcomes:
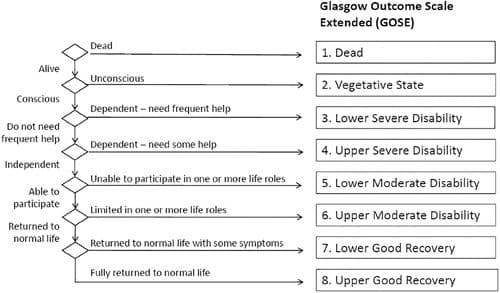
- Primary: Survival with a favorable functional outcome at 6 months assessed using the Glasgow Outcome Scale – Extended (GOS-E).
-
Secondary
- Death within 24 hours, 28 days and 6 months after injury.
Intervention: TXA 1 g bolus followed by 1 gm over 8 hours.
Control: Equivalent volume of 0.9% saline administered as bolus and infusion over 8 hours.
Design: International, randomized, double-blind, placebo-controlled study
Excluded:
- Known or suspected to be pregnant.
- Resides in a facility for older persons.
Primary Results
- Patients enrolled from 15 emergency medical services at 21 hospitals across 3 countries over 7 years.
-
1310 patients were recruited and randomized.
- 24.1% with laboratory evidence of coagulopathy.
- 39.3% of patients with head/neck injury of more than moderate severity.
- Majority of patients (92%) had blunt trauma.
- SBP < 75 mm Hg: 39%
- SBP 76-89 mm Hg: 33%
Critical Findings:
- No statistically significant difference in safety outcomes (DVT, PE, MI, Stroke)
| TXA | Placebo | RR (95% CI) | ||
| Primary Outcome | Good Functional Outcome (6 months) | 53.7% | 53.5% | 1.00 (0.9 – 1.12) |
| Secondary Outcome | Mortality 24h | 9.7% | 14.1% | 0.69 (0.51-0.94) |
| Mortality 28d | 17.3% | 21.8% | 0.79 (0.63-0.99) | |
| Mortality 6mo | 19.0% | 22.9% | 0.83 (0.67-1.03) |
Strengths:
- Asked a clinically important, patient-centered question focusing on advanced trauma systems to see if prior results in less developed systems apply more broadly.
- Multicenter, multinational study increasing external validity.
-
Randomization and blinding were appropriately conducted.
- All trial personnel were unaware of trial-group assignments.
- Broad inclusion (and limited exclusion) criteria increasing external validity.
- Demographics were well balanced in the two groups.
- Patients were actively screened for DVT (all received lower extremity ultrasound on or around day 7).
- The study was funded by a government organization as opposed to a pharmaceutical company.
Limitations:
- Patients were not consecutively recruited which may introduce bias.
- The study is relatively small (CRASH2 enrolled > 20,000 patients); just 1300 patients from 21 hospitals over 7 years.
- Protocol violations occurred in 32.7% of patients in the TXA group and 37% in the placebo group (majority was receipt of open-label TXA). This may dilute any effect of TXA.
- Outcome assessment was performed by phone interview which may be less accurate than an in person assessment.
- Data missing on 13% of patients due to loss to follow up.
Discussion:
- This is an excellently done study with strong methods.
-
The bar may be set too high in this study.
- Expecting a one time dosing of a drug to have a significant impact on 6 month outcomes may be too big an ask. TXA’s role is in immediate clot stabilization and, thus, a short-term outcome may be more relevant.
- Many trauma specialists argue that short-term mortality is a more meaningful outcome and we see improved mortality at 24 hours and 28 days.
- We should expect TXA to help in immediate stabilization and allow trauma teams the time to intervene (whether that be continued resuscitation, interventional or operative procedures).
- On the flip-side, increased survival with poor neurologic outcome is not the ideal result.
- Importantly, this study also found that TXA doesn’t increase the risk of venous or arterial thrombotic events.
- The application of these results are reserved to advanced trauma systems.
Authors Conclusions: “Among adults with major trauma and suspected trauma-induced coagulopathy who were being treated in advanced trauma systems, prehospital administration of tranexamic acid followed by an infusion over 8 hours did not result in a greater number of patients surviving with a favorable functional outcome at 6 months than placebo.”
Our Conclusions: We agree with the author’s conclusions: there was no improvement in 6-month functional outcomes in patients receiving TXA. (who are being treated in advanced trauma systems).
Potential to Impact Current Practice: It is unlikely this data will change much in terms of clinical management. Those who were skeptical of the drug’s utility will continue to be skeptical and will have their skepticism bolstered by these results. Those who were convinced that administration of TXA should be standard practice will continue to be convinced pointing to the decreased mortality and safety profile.
Bottom Line: The data on TXA in major trauma consistently demonstrates improved short-term mortality with an excellent safety profile. This should continue to make TXA part of standard trauma resuscitation while additional studies are performed.
Read More
References:
- CRASH-2 Trial Collaborators. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): A randomised, placebo-controlled trial. Lancet 2010; 376(9734): 23-32. PMID: 20554319
Post Peer Reviewed By: Salim R. Rezaie, MD (Twitter: @srrezaie) and Mark Ramzy (Twitter: @MRamzyDO)
The post Rethinking the Role of TXA: Are We Asking Too Much? appeared first on REBEL EM - Emergency Medicine Blog.
