
Background Information:
 It is well documented throughout the literature that critically ill patients admitted to the intensive care unit (ICU) with acute kidney injury have a higher morbidity and mortality.1–4 Acute kidney injury may be complicated by acidosis, hyperkalemia and other major metabolic disorders and thus the initiation of renal replacement therapy (RRT) is generally considered beneficial in these patients.5 In patients without these complications, the timing of when to initiate RRT remains unclear and is frequently debated.
It is well documented throughout the literature that critically ill patients admitted to the intensive care unit (ICU) with acute kidney injury have a higher morbidity and mortality.1–4 Acute kidney injury may be complicated by acidosis, hyperkalemia and other major metabolic disorders and thus the initiation of renal replacement therapy (RRT) is generally considered beneficial in these patients.5 In patients without these complications, the timing of when to initiate RRT remains unclear and is frequently debated.
There are three trials to know before getting to this one: ELAIN, IDEAL and AKIKI. The ELAIN trial was the only one of the three to show reduced 90-day mortality with early vs delayed initiation of RRT and was the smallest in sample size.6 The IDEAL trial concluded that early planned initiation of dialysis in stage V chronic kidney disease was not associated with improvement in survival or clinical outcomes.7 Lastly, the AKIKI trial found no significant difference with regard to mortality between an early and delayed strategy of RRT and actually saw an appreciable number of patients avert the need for RRT in a delayed strategy.8 The authors of the following study sought to investigate whether an accelerated strategy for RRT would result in lower risk of death from any cause at 90 days when compared to a standard strategy of RRT initiation.
Paper: The STARRT-AKI Investigators. Timing of Initiation of Renal-Replacement Therapy in Acute Kidney Injury. N Engl J Med. 2020; PMID: 32668114
Clinical Question:
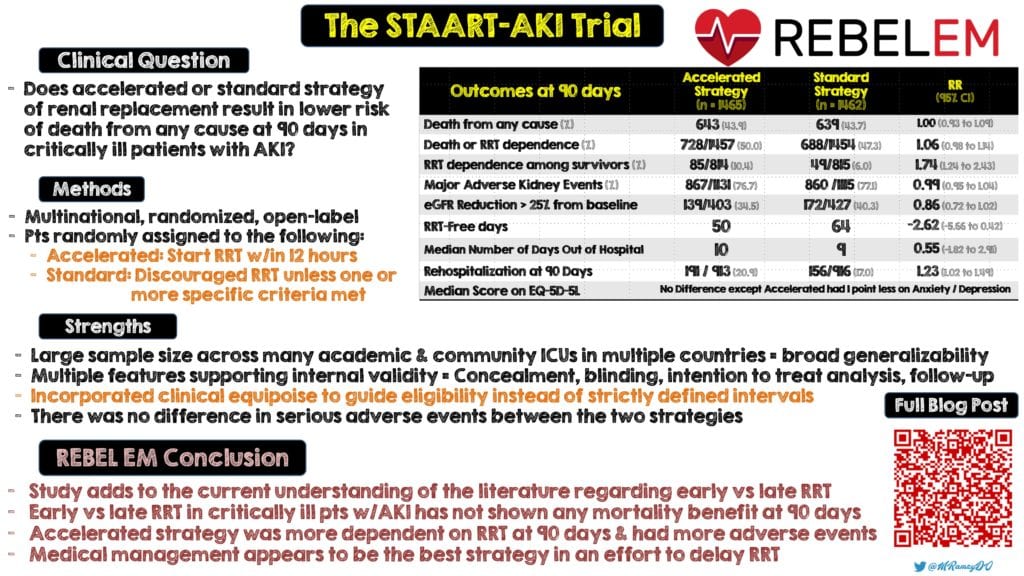
- Does initiation of an accelerated or standard renal replacement therapy result in lower risk of death from any cause at 90 days in critically ill patients with acute kidney injury?
What They Did:
- Multinational, randomized, open-label controlled ICU trial
- STARRT-AKI = Standard versus Accelerated initiation of Renal-Replacement Therapy in Acute Kidney Injury
- Occurred throughout 168 hospitals across 15 countries which included Australia, Canada, France, United Kingdom and the United States
- Patients were randomly assigned to one of the following two groups:
- Accelerated Strategy: Clinicians were to start RRT as soon as possible and within 12 hours after patients had met full eligibility criteria.
-
Standard Strategy: Clinicians were discouraged to start RRT unless development of one or more of the following:
- Serum Potassium Level > 6.0 mmol or more per liter
- pH of 7.20 or less
- Bicarbonate level of 12 mmol per liter or less
- Evidence of severe respiratory failure based on PaO2:FiO2 < 200 or less AND clinical perception of volume overload
- Persistent AKI for least 72 hours after randomization
- Discontinuation of RRT occurred at time of recovery of kidney function, withdrawal of life-sustaining support or death
- Quality of life outcomes were assessed using the European Quality of Life – 5- Dimensions 5-Level (EQ-5D-5L) questionnaire with scores ranging from 0 to 100. The higher the score the better the quality of life
Inclusion Criteria:
- Patients 18 years or older AND admitted to an ICU with kidney dysfunction defined as
- Creatinine > 1.13 mg/dL in women
- Creatinine > 1.47 mg/dL in men
- Severe acute kidney injury defined by Kidney Disease Improving Global Outcomes (KDIGO) classification as
- Doubling of creatinine level from baseline
- Serum Creatinine > 4 mg/dL or more with an increase in 0.3 mg/dL from baseline
- Urine output of less than 6 mL/kg during the preceding 12 hours
Exclusion Criteria:
- Patients who had any of the following were excluded:
- Previous renal replacement therapy in the past two months
- Kidney transplant in the past year
- Advanced chronic kidney disease (ie. GFR < 20 mL/min)
- Potassium > 5.5 mmol/L
- Bicarbonate > 15 mmol/L
- Presence of a drug overdose or dialyzable toxin that necessitates RRT
- Lack of commitment to RRT
- Presence or strong clinical suspicion of renal obstruction, rapidly progressive glomerulonephritis, vasculitis, acute interstitial nephritis or thrombotic microangiopathy
Outcomes:
Primary
- Death from any cause at 90 days after randomization
Secondary
- Major Adverse Kidney Event (MAKE) = Death, dependence on RRT or a sustained reduction in kidney function (estimated GFR < 75% of baseline value)
- Death in the ICU at 28 days or during hospitalization
- Number of days free of RRT at 90 days
- Number of ventilator and vasoactive-free days at 28 days
- Length of hospitalization
- Hospitalization free at 90 days
- Hospital related quality of life assessed using EQ-5D-5L questionnaire
Adverse Events:
- Related to RRT and vascular access through 14 days
- Serious adverse events
Results:
- Renal replacement therapy was initiated at median of 6.1 hours in the accelerated strategy group and a median of 31.1 hours in the standard strategy group
- At time of initiation, SOFA score, serum creatinine level, BUN level and positive fluid balance were higher in the standard strategy group than the accelerated strategy group
Critical Results:
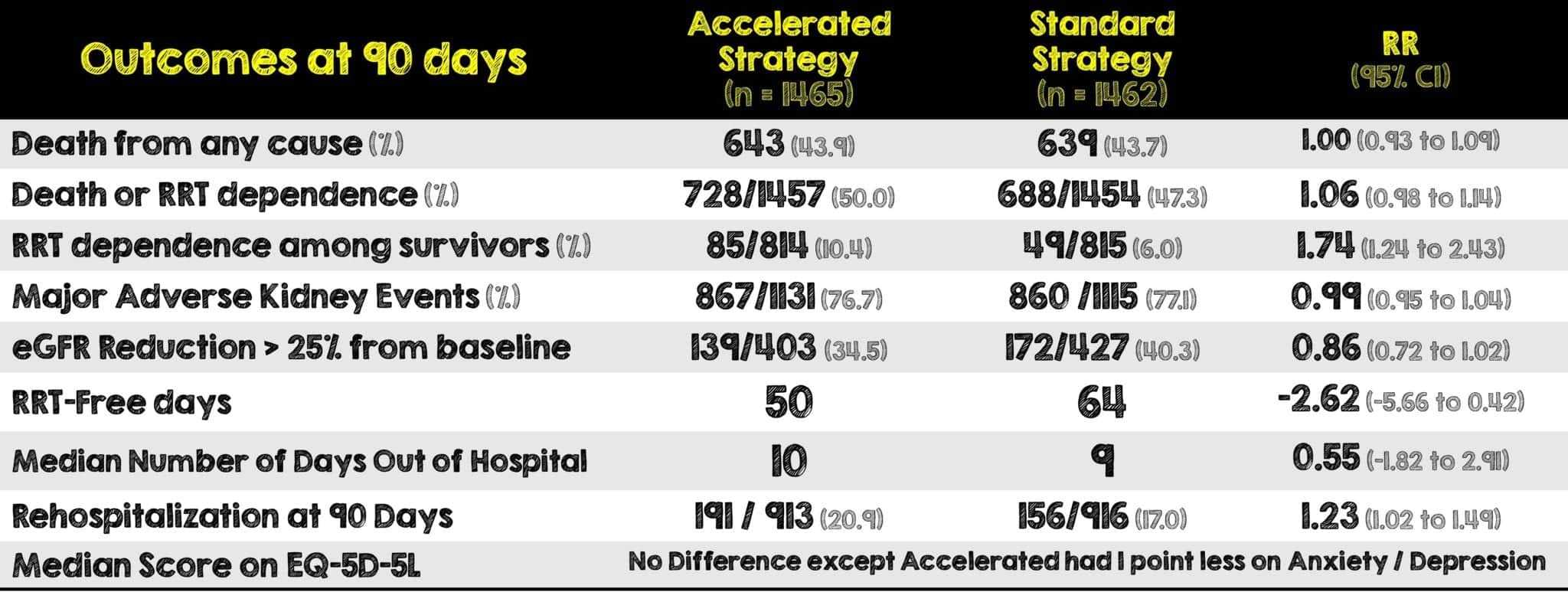
- Median length of hospital stay was 28 for survivors and 8 for non-survivors in the accelerated strategy and 29 for survivors and 9 for non-survivors in the standard strategy
- Median length of ICU stay was 9 for survivors and 7 for non-survivors in the accelerated strategy and 10 for survivors and 7 for non-survivors in the standard strategy
- The median number of ventilator free days at 28 days was 13 for the accelerated group and 12 for the standard group. Similarly, the median number of vasoactive free days at 28 days was 21 for the accelerated group and 20 for the standard group
- The median number of days out of the ICU at 28 days was 8 for the accelerated strategy and 4 for the standard strategy
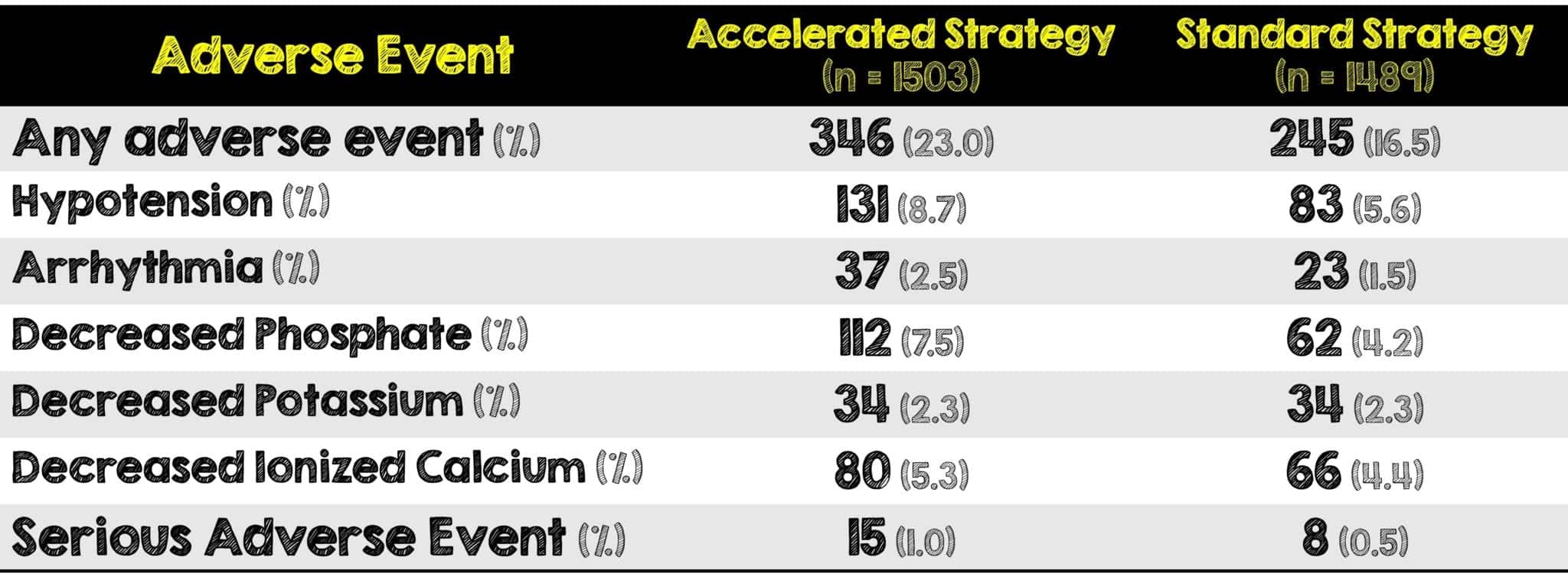
- A larger percentage of patients in the accelerated strategy had adverse events compared to the standard therapy
Strengths:
- Another large RCT in the current literature that adds more information regarding when to start RRT in critically ill patients with AKI.
- Large sample size which helps when trying to detect a clinically important difference in mortality between these two well balanced groups
- The primary outcome was corroborated with subgroup, adjusted and sensitivity analyses giving it increased validity
- Study conducted across a wide spectrum of academic and community ICUs in hospitals across multiple countries allows for broad generalizability
- Multiple features supporting internal validity such as allocation concealment, blinding, an intention-to-treat analysis and near-complete follow-up
- Those funding the study were not involved in the design, implementation, trial management, data analysis or publication
- Looked for clinically meaningful and important patient-oriented secondary outcomes such dependence on RRT and overall quality of life.
- Used multiple scoring systems such as Simplified Acute Physiology Score (SAPS) II, SOFA Scale and EQ-5D-5L to evaluate patient’s baseline and quality of life
- Incorporated clinical equipoise to guide eligibility instead of using strictly defined intervals after the patients fulfilled consensus criteria for severe AKI
- Provided clinicians evidence-based recommendations for the prescription of RRT
- Adverse events related to RRT were reviewed by the co-chairs of the trial and chair of the data and safety monitoring board within 48 hours after notification
Limitations:
- Actively discouraging physicians from initiating RRT until certain criteria are met is technically not a “standard” strategy and may serve to introduce a bias
- Patients requiring emergency RRT or those who were likely to have imminent recovery of kidney function were excluded. As a result, more than 7000 patients were not included.
- Large number of unaccounted variables that may have further added to patient heterogeneity and/or skewed the secondary outcomes
Discussion:
- This was a well done, large multicenter ICU-based RCT across many countries with the sole purpose of identifying the most appropriate timing for RRT initiation however there are important factors that must be considered.
- Death at 90 days due to any cause is not as helpful than ascertaining what adverse effects (that may or may not be related to RRT) ultimately led to that patient’s demise.
- Clinicians in the standard therapy arm were not obligated to start RRT. The discretion they had to initiate it at any time, including if they perceived deferral was no longer in the patient’s best interests, adds more subjectivity and possibly introduces more patient heterogeneity into the trial
- Interestingly, a larger percentage of patients in the accelerated strategy were dependent on RRT at 90 days and had adverse events. The authors suggest that the longer exposure to RRT may compromise kidney repair and return to baseline function.
- Hypotension and hypophosphatemia were the most common adverse events with a significant between-group difference. There was no difference in serious adverse events between the two strategies.
- With a wide variety of primary diagnoses in this study, it’s important to consider that the natural progression of disease and it’s subsequent AKI could have impacted the secondary outcomes rather than just the RRT
- Although in this study the authors state they did on observe any evidence of heterogeneity, this was only limited to illness severity and geographic region. There are still a large number of unaccounted confounding variables that may affect heterogeneity (ie. seasonality, Medication compliance, severity of comorbidities, other pre-existing conditions such as stroke, etc)
- CORRECTION MADE: During review of the data, the authors discovered a patient who was initially reported as alive at 90 days was confirmed to have died at 83 days after randomization. This error reportedly affected numbers in the results section but did not alter the main findings of the study.
- This study now serves as the third RCT that shows no significant difference in mortality between early RRT initiation and standard or delayed initiation. It’s possible that the large heterogeneity of these patients and the multiple confounding variables may be contributing to the negative result of all these studies. Future studies should not investigate when initiation of RRT is most appropriate but rather in whom early vs late RRT initiation is most appropriate.
Author’s Conclusions:
- Among critically ill patients with acute kidney injury, an accelerated renal-replacement strategy was not associated with a lower risk of death at 90 days than a standard strategy.
Our Conclusion:
- With this being yet another RCT, the decision to initiate early RRT versus a standard therapy in critically ill patients with acute kidney injury has not shown any mortality benefit at 90 days. Patients in the accelerated arm were more dependent on RRT at 90 days and had more adverse events. Multiple studies have validated these findings and short of acute indications for RRT, medically managing these patients appears to be the best strategy in an effort to delay RRT.
Clinical Bottom Line:
- This study adds to the current understanding of the literature that the initiation of early renal replacement therapy in critically ill patients with acute kidney injury did not show any mortality benefit at 90 days when compared to a standard initiation strategy.
Summary Infographic By Mark Ramzy, DO (@MRamzyDO)
For More on This Topic Checkout:
- The Bottom Line: Standard versus Accelerated Initiation of Renal Replacement Therapy
REFERENCES:
- Bonventre JV. Dialysis in Acute Kidney Injury — More Is Not Better. N Engl J Med. 2008; PMID: 18492868
- Uchino S, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005; PMID: 16106006
- Schiffl H, Lang SM, Fischer R. Daily Hemodialysis and the Outcome of Acute Renal Failure. N Engl J Med. 2002; PMID: 11821506
- RENAL Replacement Therapy Study Investigators, Bellomo R, et al. Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med. 2009; PMID: 19846848
- The STARRT-AKI Investigators. Timing of Initiation of Renal-Replacement Therapy in Acute Kidney Injury. N Engl J Med. 2020; PMID: 32668114
- Zarbock A, et al. Effect of Early vs Delayed Initiation of Renal Replacement Therapy on Mortality in Critically Ill Patients With Acute Kidney Injury: The ELAIN Randomized Clinical Trial. JAMA. 2016; PMID: 27209269
- Cooper BA, et al. A Randomized, Controlled Trial of Early versus Late Initiation of Dialysis. N Engl J Med. 2010; PMID: 20581422
- Gaudry S, et al. Initiation Strategies for Renal-Replacement Therapy in the Intensive Care Unit. N Engl J Med. 2016; PMID: 28577734
Post Peer Reviewed By: Salim R. Rezaie, MD (Twitter: @srreziae)
The post STARRT-AKI Trial: Timing of Renal Replacement Therapy Initiation in Acute Kidney Injury appeared first on REBEL EM - Emergency Medicine Blog.
