
 Background:
Background:
One of the most common treatments in critically ill patients is the administration of intravenous fluids. Historically, 0.9% saline has been one of the most common solutions used in resuscitation. In 2015 we saw the publication of the SPLIT trial, which was a randomized clinical trial of over 2200 patients in 4 ICUs in New Zealand comparing 0.9% saline (NS) vs Plasma-Lyte. This trial had many issues including, >70% of patients coming from the OR, only 15% coming from the ED, and only 4% with sepsis. The biggest issue with this trial was that the majority of patients only received 1 to 2L of fluids, making it unclear if larger volumes of unbalanced crystalloid would have worsened morbidity and mortality. In 2018, we had the publication of the SALT-ED and SMART trials.
These were both, very large, single center RCTs in the ED and ICU, respectively. Both trials used a composite outcome of MAKE 30 (Major Adverse Kidney Events at 30 Days), which is a combined outcome of unequal events (i.e. Mortality ≠ Renal Replacement Therapy). The biggest benefit of balanced crystalloids in this trial was seen in the subset of patients with sepsis (Mortality 29.4% vs 25.2%; NNT = 24). None of the previous evidence to date has helped determine a difference between Lactated Ringers Solution vs Plasma-Lyte A, nor provide guidance on patients with traumatic brain injury. We now have two more large trials published to help give us more guidance.
The Inclusion and Exclusion Criteria Were the Same In Both Trials:
Inclusion:
- Admitted to ICU
- Needed at least 1 fluid expansion (at the discretion of the attending physician)
- Not expected to be discharged the next day after enrollment
- Met at least 1 of the following criteria for acute kidney injury
- >65 years of age
- Hypotension (MAP <65mmHg, SBP <90mmHg, or vasopressor use)
- Sepsis (defined as suspected or confirmed infection plus acute organ dysfunction)
- Required mechanical ventilation or noninvasive mechanical ventilation (including HFNC) for at least 12hrs
- Early signs of kidney dysfunction (oliguria [urine output <0.5mL/kg/h for ≥3hrs] or serum creatinine level >1.2mg/dL for women or >1.4mg/dL for men)
- Liver cirrhosis or acute liver failure
Exclusion:
- Acute kidney injury who required or expected to require renal replacement therapy within 6hrs after admission
- Severe electrolyte disturbance (serum sodium ≤120mmol/L or ≥160mmol/L)
- Death considered imminent within next 24 hours
- Suspected or confirmed brain death
- Receiving palliative or comfort care only
- Previously enrolled in trial
- Thankfully removed hyperkalemia (serum potassium level >5.5mEq/L) as exclusion criteria after 2nd interim analysis
Paper #1: Type of Crystalloid – Plasma-Lyte 148 vs 0.9% Saline Solution
Paper:
Zamperi FG et al. Effect of Intravenous Fluid Treatment with a Balanced Solution vs 0.9% Saline Solution on Mortality in Crtically Ill Patients: The BaSICS Randomized Clinical Trial. JAMA 2021. [Epub Ahead of Print]
Clinical Question:
In ICU patients requiring IV fluid challenges, does the use of a balanced solution compared with saline solution (0.9% sodium chloride) improve 90-day survival?
What They Did:
- Balanced Solutions in Intensive Care Study (BaSICS)
- Double-blind, factorial, randomized clinical trial conducted at 75 ICUs in Brazil
- Factorial design:
- Assessed both effects of fluid type (reported here) and compared 2 different infusion speeds during fluid challenges (reported in second publication)
- Patients randomized to:
- Balanced solution (Plasma-Lyte 148)
- Saline solution
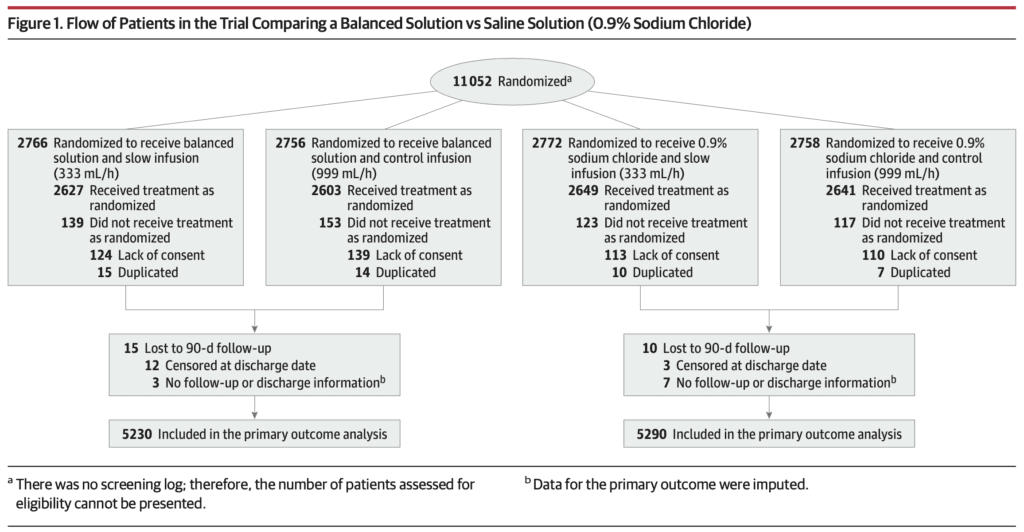
Outcomes:
- Primary: 90d mortality
-
Secondary:
- Need for renal replacement therapy up to 90d after enrollment
- Occurrence of acute kidney injury at days 3 and 7
- SOFA score at days 3 and 7
- Individual components of SOFA score at days 3 and 7
- Number of days not requiring mechanical ventilation within 28d
- Tertiary:
- ICU and hospital mortality
- ICU and hospital length of stay
Results:
- 11,052 patients randomized (532 excluded after randomization)
- 10,520 patients available for analysis
- Planned surgical admissions = 48.4% of all patients
- 68% of all patients received a crystalloid fluid bolus before ICU admission (>45% received >1L)
- 6% had hypotension and/or vasopressor use
- 3% required mechanical ventilation at enrollment
- Patients in both groups received a median of 1.5L of fluid during the 1st day after enrollment
- Accumulated median fluid administration (including study fluid and non-study fluid) during the 1st 3d after enrollment was 4.1L
- Median study fluid administered during eh same period was 2.9L
- 90d Mortality:
- Balanced Solution: 26.4%
- Saline Solution: 27.2%
- aHR 0.97; 95% CI 0.90 to 1.05; p = 0.47
- No unexpected treatment-related severe adverse events in either group
- Only two statistically significant secondary outcomes:
- SOFA Score at day 7
- Mostly due to a higher neurologic SOFA score >2 at day 7
- Subgroup Analysis of 90d Mortality:
- TBI:
- Balanced Solution: 31.3%
- Saline Solution: 21.1%
- HR 1.48; 95% CI 1.03 to 2.12
- No TBI:
- Balanced Solution: 26.2%
- Saline Solution: 27.5%
- HR 0.96; 95% CI 0.89 to 1.03
- TBI:
Strengths:
- Large, multicenter RCT
- Asks a clinically important question
- Fluids were supplied in identical 500mL bags ensuring blinding
- Physicians, patients, and individuals who assessed outcomes were blinded to assigned treatment
- Followed protocol adherence at specific time points
- Patient characteristics were well balanced between groups
Limitations:
- Large portion of patients received a fluid bolus prior to ICU admission. This could cause some contamination in the results
- Large number of surgical admissions to the ICU which could have reduced the overall trial mortality, as these patients may not be as sick as patients presenting from the ED with septic shock, etc… (Estimated trial mortality was ≈35%)
- Goal was a HR 0.90 which may have been overly ambitious for a small intervention
- Only Plasma-Lyte 148 was used for balanced solution. It is unclear if other solutions (i.e. Lactated Ringers Solution) with different buffers would provide similar results
- Overall, patients received relatively small amounts of fluid, which could be the reason we see neutral results of this study
Discussion:
- None of the secondary or subgroup analyses demonstrated benefit with the use of balanced solutions. There was a signal of possible harm for patients in the balanced solution group with a traumatic brain injury and a worse neurological SOFA component score at day 7. This could be due to measurement error (suboptimal to assess GCS in sedated patients)
Author Conclusion:
“Among critically ill patients requiring fluid challenges, use of a balanced solution compared with 0.9% saline solution did not significantly reduce 90-day mortality. The findings do not support the use of this balanced solution.”
Paper #2: Rate of Crystalloid – 333mL/hr vs 999mL/hr
Paper:
Zampieri FG et al. Effect of Slower vs Faster Intravenous Fluid Bolus Rates on Mortality in Critically Ill Patients: The BaSICS Randomized Clinical Trial. JAMA 2021. [Epub Ahead of Print]
Clinical Question:
Does a slower infusion rate compared with a control rate affect 90-day survival of critically ill patients requiring fluid challenges?
What They Did:
- Unblinded, randomized factorial clinical trial in 75 ICUs in Brazil
- Patients randomized to:
- Slower Rate: 333ml/hr
- Control Group: 999mL/hr
Outcomes:
- Primary: 90d mortality
-
Secondary:
- Need for renal replacement therapy up to 90d after enrollment
- Occurrence of acute kidney injury at days 3 and 7
- SOFA score at days 3 and 7
- Individual components of SOFA score at days 3 and 7
- Number of days not requiring mechanical ventilation within 28d
- Tertiary:
- ICU and hospital mortality
- ICU and hospital length of stay
Results:
- 10,520 patients analyzed
- Patients assigned to slower rate received a mean of 1162mL on 1st day
- Patients assigned to control group received a mean of 1252mL on 1st day
- Unplanned admissions accounted for 51.2% of patients receiving the slower infusion rate and 52.0% receiving the control infusion rate
- 9% receiving slower infusion and 61.2% in the control group were either hypotensive or using vasopressors at enrollment
- 90d Mortality:
- Slower Rate: 26.6%
- Control Group: 27.0%
- aHR 1.03; 95% CI 0.96 to 1.11; p = 0.46
- No significant interaction between fluid type and infusion rate (p = 0.98)
- On day 3, patients in the slower infusion group did better than control group with a lower
- SOFA Score (Difference =0.10; 95% CI -0.21 to -0.01)
- Lower frequency of hemodynamic SOFA score of more than 2 (marking a lower use of vasopressors) (32.5% vs 35.3%; OR 0.89; 95% CI 0.80 to 0.98)
- Frequency of respiratory SOFA score of more than 2 (marking a lower frequency of being mechanically ventilated with a P/F ratio less than 200%) (6.2% vs 7.5%; OR 0.81; 95% CI 0.68 to 0.97)
- None of these differences, however, were sustained on day 7
- Frequency of coagulation SOFA score of more than 2 was higher on day 3 for the slower infusion group (4.7% vs 3.9%; OR 1.31; 95% CI 1.05 to 1.64)
- None of these differences, however, were sustained on day 7
- No reported unexpected severe adverse events in either group
Strengths:
- Large, multicenter RCT
- Asks a clinically important question
- Baseline features were well balanced between groups
- Most fluid challenges were performed in the assigned rate o measured days (>90% of all fluid challenges on day 1 done at the assigned infusion rate)
Limitations:
- Assessment of fluid infusion was unblinded (patients and physicians were aware of the groups to which they were allocated)
- Large portion of patients received a fluid bolus prior to ICU admission. This could cause some contamination in the results
- Overall, patients received relatively small amounts of fluid, which could be the reason we see neutral results of this study
- Slower infusion rate was defined arbitrarily at 333mL/hr, not based on previous evidence (333mL/hr not that slow)
- Secondary endpoints were not adjusted for multiple comparisons and therefore only hypothesis generating
- The reason for fluid bolus challenges were not recorded
- No recording of immediate effects of fluid challenges on hemodynamic parameters
Author Conclusion:
“Among patients in the intensive care unit requiring fluid challenges, infusing at a slower rate compared with a faster rate did not reduce 90-day mortality. These findings do not support the use of a slower infusion rate.”
Clinical Take Home Point:
- Among critically ill patients, requiring fluid challenges, the type of fluid (balanced solution (Plasma-Lyte 148) compared with 0.9% saline solution) nor the rate of fluid administration (333mL/hr vs 999mL/hr) did not result in improved 90-day mortality. These findings do not support the use of balanced solutions over 0.9% saline solution or the use of a slower infusion rate.
- The type and the rate of crystalloid infusion may not be as important as the amount of crystalloid given when given in smaller volumes (<2 – 3L). Although, still unclear, when larger volumes are given (i.e. >3L of fluid), consider sticking with balanced crystalloids
References:
- Zamperi FG et al. Effect of Intravenous Fluid Treatment with a Balanced Solution vs 0.9% Saline Solution on Mortality in Crtically Ill Patients: The BaSICS Randomized Clinical Trial. JAMA 2021. [Epub Ahead of Print]
- Zampieri FG et al. Effect of Slower vs Faster Intravenous Fluid Bolus Rates on Mortality in Critically Ill Patients: The BaSICS Randomized Clinical Trial. JAMA 2021. [Epub Ahead of Print]
For More Thoughts on This Topic Checkout:
- EMCrit: Breaking News on Fluid Choice and Rate – The BaSICS Trial
- REBEL EM: Is the Great Debate Between Balanced vs Unbalanced Crystalloids Finally Over?
- First10EM: The BaSICS Trial – Normal Saline Has Been Fine All Along
- The Bottom Line: BaSICS
- St. Emlyn’s Blog: The BASICS Trial. 0.9% Saline vs Balanced Solution. Does it Matter?
Post Peer Reviewed By: Anand Swaminathan, MD (Twitter: @EMSwami)
The post The BaSICS Trial – Balanced Solution vs 0.9% Saline Solution in Critically Ill Patients appeared first on REBEL EM - Emergency Medicine Blog.
