 Background: The COVID-19 pandemic has been the focus of massive research efforts over the last three years. Our understanding of the disease and effective treatments to reduce mortality have progressed rapidly during this time. However, the medical community is only just starting to understand long-COVID (WHO Definition: the continuation or development of new symptoms 3 months after the initial SARS-CoV-2 infection, with these symptoms lasting for at least 2 months with no other explanation). While it is unclear how prevalent long-COVID is, if it affects even a small percentage of patients, we will be faced with caring for millions of patients due the very high incidence of COVID. Investigation into preventive treatments is critically important.
Background: The COVID-19 pandemic has been the focus of massive research efforts over the last three years. Our understanding of the disease and effective treatments to reduce mortality have progressed rapidly during this time. However, the medical community is only just starting to understand long-COVID (WHO Definition: the continuation or development of new symptoms 3 months after the initial SARS-CoV-2 infection, with these symptoms lasting for at least 2 months with no other explanation). While it is unclear how prevalent long-COVID is, if it affects even a small percentage of patients, we will be faced with caring for millions of patients due the very high incidence of COVID. Investigation into preventive treatments is critically important.
Article: Bramante CT et al. Outpatient treatment of COVID-19 and incidence of post-COVID-19 condition over 10 months (COVID-OUT): a Multicentre, randomised, quadruple-blind, parallel-group, phase 3 trial. Lancet Infect Dis 2023 PMID: 37302406
Clinical Question: Does outpatient treatment with ivermectin, fluvoxamine or metformin soon after SARS-CoV-2 infection reduce the risk of developing long-COVID in overweight patients?
Population: Adults aged 30-85 years of age with BMI > 25 (> 23 in those identifying as Asian or Latino) who had COVID-19 symptoms for fewer than 7 days and a documented SARS-CoV-2 positive PCR or antigen test within 3 days of enrollment without prior SARS-CoV-2 infection. Participants were recruited remotely through online advertising, patient portal messages and health-system advertising. Prior vaccination was not an exclusion criteria.
Outcomes:
- Primary: The development of long-COVID up to 300 days after enrollment. This secondary outcome became the primary outcome (see discussion for more detail).
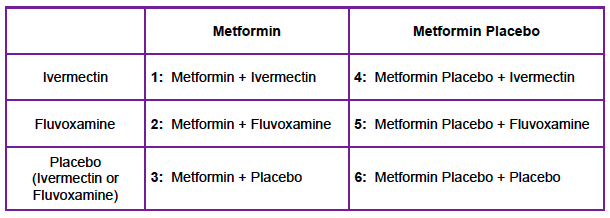
Intervention: 2 x 3 factorial design (see table below)
- Metformin 500mg qD on day 1, 500mg BID on days 2 to 5, then 500mg qAM and 100mg qHS up to day 14
- Ivermectin 390 to 470ug/kg/d x3d
- Fluvoxamine 50mg qD x1d followed by 50mg BID up to day 14
Control: Identical placebo pills
Design: Multicenter, randomized, quadruple-blinded, parallel group, phase 3 study.
Excluded:
- Patients already taking one of the study medications
Primary Results
-
- Enrolled: 1431 patients
- 1126/1323 participants in the modified, intention-to-treat population were consented for long-term follow up and completed at least one survey.
- Metformin: 564 patients
- Placebo: 562 patients
- Median BMI ≈30kg/m2
- Vaccination Status
- SARS-CoV-2 Primary Vaccination ≈55% of enrolled patients
- SARS-CoV-2 Booster Vaccination ≈5% of enrolled patients
- Median time from symptom onset to study drug initiation =5d
- SARS-CoV-2 Dominant Variant at Time of Randomization was the Delta Variant in 70% of patients
- Enrolled: 1431 patients
Critical Findings:
- 93/1126 (8.3%) reported a long COVID diagnosis by day 300.
- Alpha dominant: 7.9%
- Delta dominant 8.3%
- Omicron dominant: 8.4%
- No statistically significant differences found for fluvoxamine or ivermectin.
|
Metformin (95% CI) |
Placebo (95% CI) |
Absolute Difference |
Hazard Ratio (95% CI) |
NNT |
||
|
Long COVID |
6.3% (4.2-8.2) |
10.4% (7.8-12.9) |
4.1% |
0.59 (0.39 – 0.89) |
25 |
Statistically Significant Difference |
Strengths:
- The study asks an important, patient centered question.
- Broad inclusion criteria increasing external validity.
- Pregnant/lactating patients were not excluded.
- Blinding procedures were well constructed as to mask group allocation.
- Robust patient follow-up process tailored to the patient’s preference.
- Had a very precise four-part definition of severe COVID-19 (Hypoxemia at home, ED Visit, Hospitalization, death)
- 95% of participants completed at least 9 months of follow-up
- Patient had to receive diagnosis of long-COVID from a medical professional (not simply based on symptom reporting).
Limitations:
- Enrollment was investigator initiated which may introduce bias.
- Relatively small study given the scope of the disease.
- This trial excluded groups at low risk of severe COVID-19—adults with a normal BMI and those who were younger than 30 years—and whether these findings would be generalisable to those populations remains unknown
Discussion
- This is the first, high-quality study showing that the incidence of long-COVID can be reduced through a medical intervention.
- This study helps to solidify the diagnosis of long COVID; for an effective therapy to exist, there must also exist a disease (see Faust editorial linked below).
- The original trial investigated the rate of severe COVID-19 by day 14. As a secondary outcome, the authors wanted to investigate the development of long COVID.
- On the face of it, this appears to be publication of a secondary outcome and the authors state as much.
- However, it is slightly more complicated: When the authors became aware of the phenomenon of long COVID, they reconsented patients into this separate study prior to any analysis of data or unblinding.
- Essentially, the authors adapted to a rapidly evolving situation in order to get the best possible data to influence decisions.
- The mechanism of action for metformin’s reduction in long-COVID is not well-understood. More research is necessary in this area.
- This data does not speak to whether metformin can be given after the development of long-COVID in efforts to ameliorate the disease. Future research in this area is necessary.
- This study provides additional data that vaccination reduces the risk of developing long-COVID. Among participants who had received at least the primary SARS-CoV-2 vaccine series, 41 (6.6%) of 619 reported a diagnosis of long COVID, compared with 52 (10.3%) of 507 who were unvaccinated.
Authors Conclusions: “Outpatient treatment with metformin reduced long COVID incidence by about 41%, with an absolute reduction of 4·1%, compared with placebo. Metformin has clinical benefits when used as outpatient treatment for COVID-19 and is globally available, low-cost, and safe. “
Our Conclusions: We agree with the authors. A short course of metformin reduces the risk of long COVID incidence in overweight/obese adults 30-85 years of age when started early in the course of clinical COVID-19.
Potential to Impact Current Practice: With the potential of hundreds of millions of people to be diagnosed with long COVID in the absence of treatment, metformin could have a significant impact without a large price. Just as important, this study helps to solidify long COVID as a true entity.
Bottom Line: A short course of metformin should be considered in overweight/obese patients with COVID-19 who present early in their disease course to reduce the risk of developing long COVID.
References:
- Bramante CT et al. Outpatient treatment of COVID-19 and incidence of post-COVID-19 condition over 10 months (COVID-OUT): a Multicentre, randomised, quadruple-blind, parallel-group, phase 3 trial. Lancet Infect Dis 2023 PMID: 37302406
- Faust J. The therapeutic validation of long-COVID. Lancet Infect Dis 2023. PMC: 10250006
For More Thoughts on This Topic Checkout:
Post Peer Reviewed By: Salim R. Rezaie, MD (Twitter: @srrezaie) and Mark Ramzy, DO (Twitter: @MRamzyDO)
The post The COVID-OUT Trial: Does Metformin Reduce the Risk of Long COVID? appeared first on REBEL EM - Emergency Medicine Blog.