
 Background: The use of ketamine and etomidate for induction in rapid sequence intubation is heavily debated. The Ketased Trial (Jabre 2009) reported no significant difference between the two induction agents. However, recently the National Emergency Airway Registry reported ketamine is associated with more-frequent hypotension than etomidate in critically ill patients (Mohr 2020). Another study reported an association with adrenocorticotropic hormone (ACTH) and slightly higher SOFA scores when etomidate was used, but there was no evidence of increased mortality (Bruder 2015). A recently published meta-analysis found etomidate to be the safer induction agent. (Sharda 2022)
Background: The use of ketamine and etomidate for induction in rapid sequence intubation is heavily debated. The Ketased Trial (Jabre 2009) reported no significant difference between the two induction agents. However, recently the National Emergency Airway Registry reported ketamine is associated with more-frequent hypotension than etomidate in critically ill patients (Mohr 2020). Another study reported an association with adrenocorticotropic hormone (ACTH) and slightly higher SOFA scores when etomidate was used, but there was no evidence of increased mortality (Bruder 2015). A recently published meta-analysis found etomidate to be the safer induction agent. (Sharda 2022)
Etomidate and ketamine act distinctly from each other. Etomidate works on the GABA receptor, while ketamine works as an NMDA antagonist. Yet, both agents provide the same outcome, sedation. So, which agent produces a more favorable outcome for patients? The answer remains uncertain.
Article: Matchett G et al; EvK Clinical Trial Collaborators. Etomidate Versus Ketamine for Emergency Endotracheal Intubation: A randomized Clinical Trial. Intensive Care Med. 2022. PMID: 34904190.
Clinical Question: What is the 7-day survival rate of etomidate compared to ketamine during rapid sequence intubation?
What They Did
- Prospective, randomized 1:1, parallel-assignment, open-label, single-center trial
- Randomization process: Sequentially numbered opaque, sealed envelope in blocks of 8 that directed the team to use either etomidate (0.2 to 0.3 mg/kg intravenous) or ketamine (1-2 mg/kg intravenous)
- Intubation performed by procedure-focused airway team under Department of Anesthesiology on hospital patients in Level 1 trauma center in Dallas.
- Enrolled 801 patients
- 396 patients received etomidate, and 398 received ketamine
- The study was conducted from Jul 6, 2016, to Sept 7, 2020
Population
-
Inclusions:
- Any adult 18 years or older in need of emergency endotracheal intubation
-
Exclusions:
- Children <18 years
- Pregnant
- Previously enrolled in the trial
- Requiring endotracheal intubation without sedation
- Neurologically obtunded
- Requiring awake intubation
- Known allergy to etomidate or ketamine
- Anyone that opted out of the clinical trial
Outcomes
- Primary Outcome: 7-day survival
-
Secondary Outcomes:
- 28-day survival
- Duration of mechanical ventilation
- ICU length-of-stay
- Usage and duration of vasopressor therapy
- Serial SOFA scores on days 1–4
- New diagnosis of adrenal insufficiency
-
Exploratory Outcomes:
- Post-induction cardiovascular collapse
- Post-induction vasopressor bolus
- Post-induction systolic blood pressure
- Post-induction IV fluid bolus
- Delta SBP mmHg
Results
- 1952 patients were assessed for eligibility
- 1151 patients were excluded
-
801 patients enrolled
- 400 patients were randomized to receive Etomidate
- 401 patients were randomized to receive ketamine
-
10 post-enrollment withdrawals
- 4 in etomidate arm
- 6 in ketamine arm
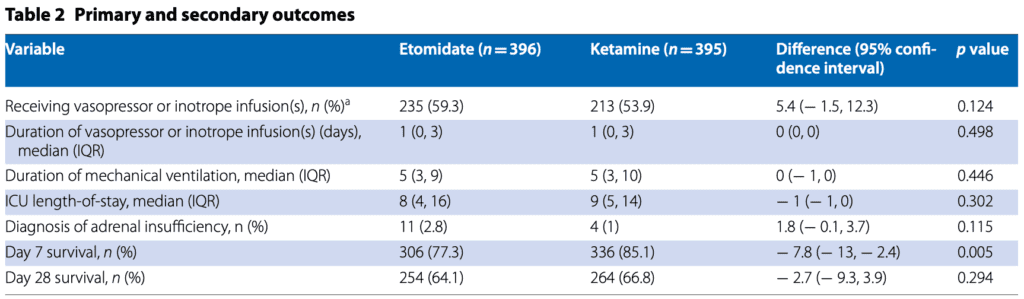
- Day 7 survival rate for ketamine was higher than etomidate.
- Day 28 survival rates for both ketamine and etomidate were not significantly different.
- Duration of mechanical ventilation, ICU, and percentage of vasopressor use were not significantly different.
- Ketamine was associated with more-frequent administration of post-induction rescue therapy with vasopressors and a greater incidence of post-induction cardiovascular collapse.
Strengths
- A large study, appropriately powered, increases generalizability.
- Broad selection criteria with limited exclusion criteria increase generalizability.
- A randomized study design increases generalizability.
- The airway team had no clinical role in managing patients after intubation.
- Induction drug doses were consistent with the doses recommended in the trial protocol.
- Groups were similar concerning neuromuscular blocking agent and dose, laryngoscope choice, or the number of intubation attempts.
- Investigators asked a heavily debated patient-focused research question.
- Outcomes were patient-oriented
- Performed an intention-to-treat analysis
Limitations:
- Single-center study affects generalizability.
- A highly specialized airway team was utilized for intubation, likely unavailable in many institutions.
- All intubations were performed on hospitalized patients, limiting application to the ED population.
- It’s unclear if all intubated patients were assessed for inclusion in the study, and the patient cohort may represent a convenience sample.
- The airway team was permitted to screen-out patients regardless if exclusion criteria were met, without any explanation.
- Airway teams could change meds if they felt a patient benefited from a specific therapy, contributing to selection bias.
-
Baseline characteristics appear unbalanced.
-
More patients in the etomidate arm had Knaus category A and were more functional at baseline than patients with other grades.
- Knaus Category:
- A = Previous good health with no functional limitations
- B = Mild to moderate limitation of activity because of a chronic medical problem
- C = chronic disease producing serious but not incapacitating limitation of activity
- D = Severe restriction of activity due to disease, including people incapacitated or institutionalized because of illness
- More patients in the etomidate arm were admitted to the SICU vs. MICU.
-
More patients in the etomidate arm had Knaus category A and were more functional at baseline than patients with other grades.
- The care team and outcome assessors were unblinded to the study arm, which may bias the results.
- The significance of 7-day survival is unclear.
- Though the outcomes are patient-centered, investigators are singularly focused on mortality, and patients will likely also care about functional status and morbidity.
- There was no information on the proportion of patients experiencing cardiovascular collapse before induction of anesthesia.
- There was no quantitative information on the amount of preexisting IV fluids and vasopressor therapies.
Discussion
- Inside the numbers:
-
- Patients receiving etomidate were 1.6x more likely to die at seven days than ketamine. But this statistically significant outcome disappears by day 28. What is the relevance of a statistically different survival outcome on day seven that converges by day 28? Are there unmeasured confounders affecting short-term outcomes but not longer-term outcomes? Or is this just statistical noise?
- Additionally, there is so much missing data and similarity between groups post-induction in terms of heart rate, blood pressure, and change in blood pressure that it is unclear why the ketamine group got more fluids/vasopressors.
- Moreover, the clinical significance of 7-day survival is unclear. Will patients care if they survived seven days but ultimately died by day 28? In what capacity will the patient be? Are they brain dead or paralyzed? There is no mention of these patients’ condition after intubation.
- We must also consider resource utilization of managing critically ill patients for additional days who are still just as likely to die a few weeks later. These extra days burden the healthcare system, which may be strapped for medicines, supplies, and hospital beds.
- Bias:
-
- Patients were not enrolled consecutively. Enrollment was at the discretion of the airway team, and patients could be excluded outside the research exclusion criteria. Additionally, the airway team could change medications during intubation, leading to selection bias. The investigators could have selected patients believed to produce the desired outcome biasing the results in one direction over another.
- Baseline characteristics were not equal. Patients in the etomidate arm had higher Knaus category A designation rates. They were more functional at baseline, favoring etomidate which is the opposite of what investigators found. Patients in the etomidate arm were admitted to SICU at higher rates. In contrast, patients in the ketamine arm had higher rates of MICU admissions. There is no information on resuscitation strategies or medications administered, and there may be drastic differences in management based on patient location and diagnosis. It’s unclear whether each study arm had the same prognosis at enrollment.
- The clinical care team was unblinded to the therapy the patients received. We know more patients in the ketamine arm experienced a post-induction cardiovascular collapse, and these patients were more likely to receive IV fluids and vasopressors. There is no information on the vasopressor agent or dosing of fluid volume. The care team could have provided more aggressive management to one group over another. This could explain the difference in 7-day survival, which was not seen at 28 days. It’s unclear if patients in each arm had the same prognosis at the end of the study.
- Outcome assessors were not blinded and could have chosen outcomes favoring one induction agent over another. Perhaps choosing another day washes away the positive findings of the 7-day survival difference, as was seen in the secondary outcome of 28-day survival.
Author’s Conclusion: “While the primary outcome of Day 7 survival was greater in patients randomized to ketamine, there was no significant difference in survival by Day 28.”
Our Conclusion:
This is an unblinded, single-center trial using an anesthesia team for intubation, with missing data and significant sources of bias. Therefore, we are skeptical of the results, and it is difficult to draw conclusions from the data presented in this paper. The debate concerning which is the superior induction agent for RSI continues.
References:
- Matchett G, Gasanova I, Riccio CA, Nasir D, Sunna MC, Bravenec BJ, Azizad O, Farrell B, Minhajuddin A, Stewart JW, Liang LW, Moon TS, Fox PE, Ebeling CG, Smith MN, Trousdale D, Ogunnaike BO; EvK Clinical Trial Collaborators. Etomidate versus ketamine for emergency endotracheal intubation: a randomized clinical trial. Intensive Care Med. 2022 Jan;48(1):78-91.Epub 2021 Dec 14. PMID: 34904190.
- Jabre P, Combes X, Lapostolle F, Dhaouadi M, Ricard-Hibon A, Vivien B, Bertrand L, Beltramini A, Gamand P, Albizzati S, Perdrizet D, Lebail G, Chollet-Xemard C, Maxime V, Brun-Buisson C, Lefrant JY, Bollaert PE, Megarbane B, Ricard JD, Anguel N, Vicaut E, Adnet F; KETASED Collaborative Study Group. Etomidate versus ketamine for rapid sequence intubation in acutely ill patients: a multicentre randomised controlled trial. Lancet. 2009 Jul 25;374(9686):293-300. Epub 2009 Jul 1. PMID: 19573904.
- Mohr NM, Pape SG, Runde D, Kaji AH, Walls RM, Brown CA 3rd. Etomidate Use Is Associated With Less Hypotension Than Ketamine for Emergency Department Sepsis Intubations: A NEAR Cohort Study. Acad Emerg Med. 2020 Nov;27(11):1140-1149. Epub 2020 Jul 20. PMID: 32602974
- Bruder EA, Ball IM, Ridi S, Pickett W, Hohl C. Single induction dose of etomidate versus other induction agents for endotracheal intubation in critically ill patients. Cochrane Database of Systematic Reviews 2015, Issue 1. Art. No.: CD010225. PMID: 25568981
- Sharda SC, Bhatia MS. Etomidate Compared to Ketamine for Induction during Rapid Sequence Intubation: A Systematic Review and Meta-analysis. Indian J Crit Care Med. 2022;26(1):108-113. PMID: 35110853
Guest Post By:

Shahrukh Syed, MD
PGY-1, Emergency Medicine Resident
Nuvance Health, Poughkeepsie, New york
Email: shahrukh.syed@my.rfums.org

Marco Propersi, DO FAAEM
Vice-Chair, Emergency Medicine
Vassar Brothers Hospital, Poughkeepsie, New York
Twitter: @marco_propersi
Post-Peer Reviewed By: Salim R. Rezaie, MD (Twitter: @srrezaie)
The post The EvK Trial: Ketamine vs Etomidate for Rapid Sequence Intubation appeared first on REBEL EM - Emergency Medicine Blog.
