
 Background: Vitamin C dissociates to ascorbate at physiological pH and is not organically synthesized by the human body. Vitamin C can function as an antioxidant and may mitigate endothelial oxidative stress in sepsis. (Amrein 2018) A small, single-center, before-and-after study utilized a sepsis cocktail consisting of IV thiamine, hydrocortisone, and vitamin C and discovered a significant decrease in mortality compared to the control group. (Marik 2017) But further randomized controlled studies have not shown the same benefit. (Fujii 2022, Sevransky 2021)
Background: Vitamin C dissociates to ascorbate at physiological pH and is not organically synthesized by the human body. Vitamin C can function as an antioxidant and may mitigate endothelial oxidative stress in sepsis. (Amrein 2018) A small, single-center, before-and-after study utilized a sepsis cocktail consisting of IV thiamine, hydrocortisone, and vitamin C and discovered a significant decrease in mortality compared to the control group. (Marik 2017) But further randomized controlled studies have not shown the same benefit. (Fujii 2022, Sevransky 2021)
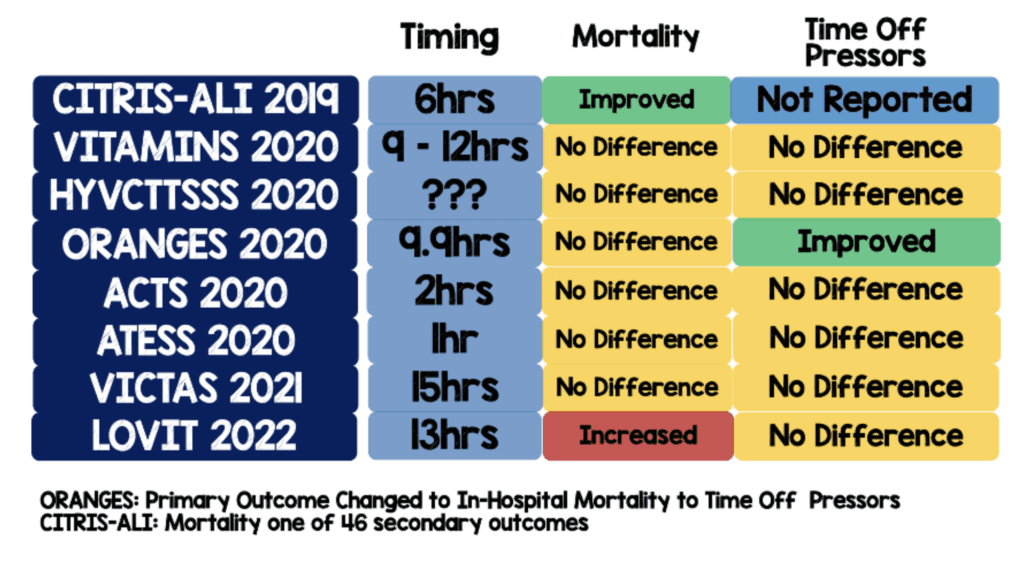
Contrary to the above trial, another RCT investigated patients in the ICU with sepsis and acute respiratory distress syndrome. Trialists gave patients 50mg/kg of vitamin C every 6 hours. Although the high-dose vitamin C did not impact short-term SOFA score or in lowering inflammatory markers, it showed a lower 28-day risk of death compared to patients that received placebo. (Fowler 2019) This concept and study design provided the framework for the Lessening Organ Dysfunction with Vitamin C (LOVIT) clinical trial.
Clinical Question: Does high-dose vitamin C improve survival and/or persistent organ dysfunction rates in septic patients in the ICU on vasopressors?
Article: Lamontagne F et al. Intravenous Vitamin C in Adults with Sepsis in the Intensive Care unit. N Engl J Med 2022. PMID: 35704292.
What They Did:
- International, multicenter, randomized, placebo-controlled, double-blinded, clinical trial
- Enrolled patients from 35 adult medical surgical ICUs in Canada, France, and New Zealand
- Patients were randomly assigned a 1:1 ratio to vitamin C or placebo
Population:
-
Inclusion:
- Patients > 18 years of age
- Admitted to the ICU ≤ 24 hours
- On vasopressors
- Proven or suspected infection as the primary diagnosis
-
Exclusion:
- Contraindications to vitamin C therapy
- Receipt of open-label vitamin C
- Expected death or withdrawal of life-sustaining therapy within 48 hours
Outcomes:
-
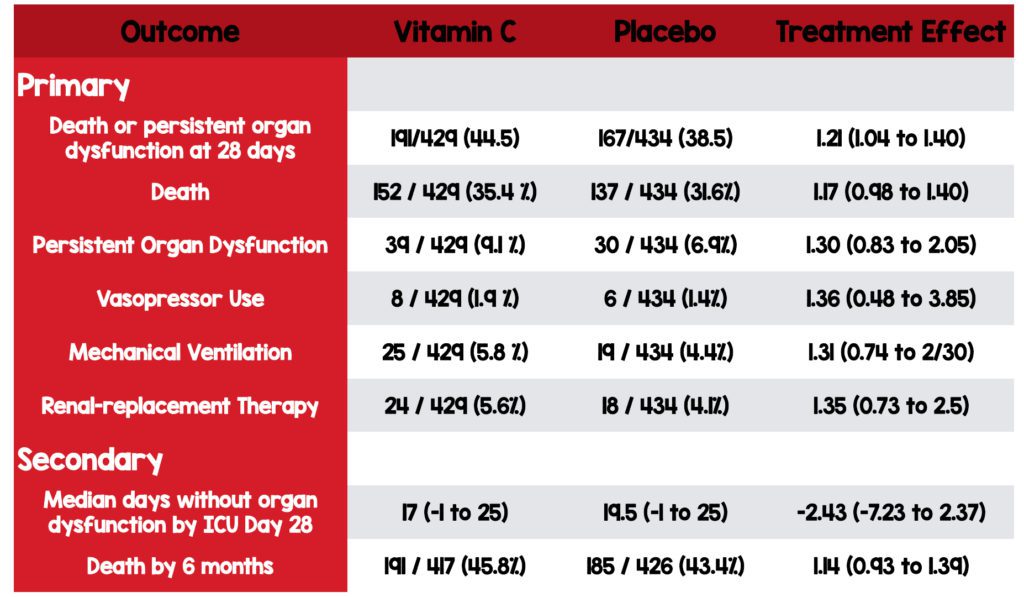
Primary: Composite Outcome
- Death
- Persistent organ dysfunction on day 28 (Vasopressors, invasive mechanical ventilation, or new renal replacement therapy)
-
Secondary:
- The Median number of days without organ dysfunction in the ICU up to day 28
- Mortality at 28 days
- Mortality at 6 months
- SOFA score at days , 2, 3, 4, 7, 10, 14, 28
- Biomarkers: lactate, IL-1ß, tnf-𝞪, thrombomodulin and angiopoietin-2
- EQ-5D-5L score at 6 months
Intervention:
- 50mg/kg dose of IV Vitamin C in 50-mL of D5 water or NS, every 6 hours for up to 96 hours (max 16 doses) while the patient is in the ICU.
Control:
- A matching placebo infusion in 50 mL of D5 water or NS.
Results:
- 2234 patients were assessed for eligibility,
-
834 excluded
- 528 were eligible but not enrolled
-
872 patients randomized
- 435 randomized to vitamin C arm
- 437 randomized to the placebo arm
- Time to randomization from ICU admission was ≈12.6hrs
- Almost 2/3rds of patients were on mechanical ventilation
Strengths:
- The study asks a patient-centered research question
- Patients, clinicians, trial personnel, and statisticians were unaware of group assignments
- The large sample size increases generalizability and external validity.
- A double-blind, randomized clinical trial design increases generalizability and external validity.
- Baseline characteristics were similar regarding clinical indicators and biomarkers.
- The use and duration of interventions and life-sustaining therapies during the course of the ICU were similar between groups.
- Median enrollment time was close to 12 hours after ICU admission
- Groups also had similar urine output and fluid balance during the first 7 days in the ICU
- Overall compliance was excellent 96% of patients received at least 90% of their doses.
- Follow up on >80% of patients.
- Used an intention-to-treat analysis for the primary outcome, resembling real-world practice instead of a per-protocol analysis.
- Performed best-case-worst-case sensitivity analysis for patients with missing data and those lost to follow-up.
Limitations:
- 528 patients were eligible but not enrolled in this trial. This makes the trial a convenience sample as opposed to a consecutive sample
- The primary outcome was a composite outcome of unequal relevance
- Analysis of biomarkers is a disease-oriented outcome
- 6 month follow-up was conducted via telephone and is subject to recall bias
- Like most trials on vitamin C in sepsis, there is lots of subgroup analysis. The more endpoints investigated, the greater the potential to discover something just by chance alone. We often refer to this as data dredging.
- Specific pathogens causing sepsis and the appropriateness of antimicrobial therapy were not collected.
- Patients in respiratory distress were not identified, so it remains unclear whether this subgroup had a different response to vitamin C.
- The patient population in this study is primarily from high-income countries. This population differs substantially from low/middle-income countries.
Discussion:
Inside the Numbers:
- Death was seen more often in the high-dose vitamin C group vs. placebo, and the number needed to harm in this study was 16. Critics will no doubt point to methods and criticize the use of vitamin C monotherapy and the absence of thiamine and corticosteroids. It bears mention that patients received vitamin C within 4 hours after randomization compared to 6 hours in the CITRIS-ALI trial. Additionally, trialists were looking for an absolute between-group difference of 10%. This is a huge difference. The larger the difference, the more difficult it will be to show a benefit.
- In the subgroups of patients with COVID, there was a trend toward benefits with vitamin C therapy. This finding may be statistical noise, but it is noteworthy and hypothesis-generating at the very least.
- Only 1 of 8 clinical trials published in the past three years evaluating the utility of high-dose Vitamin C in septic patients showed an improvement in mortality. 6 of 8 demonstrated no difference, and this trial showed increased mortality.
Composite outcomes
- Investigators used a composite for the primary outcome. Composite outcomes decrease the enrollment size required to power a study adequately. However, researchers will often group outcomes of varying clinical importance. Furthermore, the outcome is typically driven by one component over the others. For example, In this study, the composite outcome was driven by the number of patients who died. In addition, all components often do not carry the same clinical importance. For example, death is a more clinically relevant outcome than organ dysfunction.
Subgroup analysis
- Subgroup analysis is a common occurrence in clinical trials. However, clinical trials are powered to assess the primary outcome and not any findings on subgroup analysis. Therefore, subgroup analysis may provide data of interest. However, the information gleaned is purely hypothesis-generating and possibly the focus of further study. (Burke 2015)
- Subgroup analysis can lead to “false positives due to multiple comparisons, false negatives due to inadequate power, and limited ability to inform individual treatment decisions because patients have multiple characteristics that vary simultaneously.” (Burke 2015)
Authors Conclusions: “In adults with sepsis receiving vasopressor therapy in the ICU, those who received intravenous vitamin C had a higher risk of death or persistent organ dysfunction at 28 days than those who received placebo.”
Clinical Bottom Line:
This large and high-quality clinical trial delivers yet another and hopefully final blow to Vitamin C. Based on the overwhelming preponderance of high-quality evidence, there appears to be no role for Vitamin C in sepsis.
For More on This Topic, Checkout:
- St. Emelyn’s: Vitamin C and sepsis (again).
- SGEM: ALL MY LOVIT, VITAMIN C WON’T WORK FOR YOU
- First10EM: Research Roundup (August 2022)
- The Bottom Line: Lovit Trial
References:
- Amrein K, Oudemans-van Straaten HM, Berger MM. Vitamin therapy in critically ill patients: focus on thiamine, vitamin C, and vitamin D. Intensive Care Med 2018;44:1940-4. PMID: 29520660
- Marik PE, Khangoora V, Rivera R, Hooper MH, Catravas J. Hydrocortisone, vitamin C, and thiamine for the treatment of severe sepsis and septic shock: a retrospective before-after study. Chest 2017; 151:1229-38. PMID: 27940189
- Fujii T, Salanti G, Belletti A, et al. Effect of adjunctive vitamin C, glucocorticoids, and vitamin B1 on longer-term mortality in adults with sepsis or septic shock: a systematic review and a component network meta-analysis. Intensive Care Med 2022;48:16-24. PMID: 34750650
- Sevransky JE, Rothman RE, Hager DN, et al. Effect of vitamin C, thiamine, and hydrocortisone on ventilator- and vasopressor-free days in patients with sepsis: the VICTAS randomized clinical trial. JAMA 2021;325:742-50. PMID: 33620405
- Fowler AA III, Truwit JD, Hite RD, et al. Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: the CITRIS-ALI randomized clinical trial. JAMA 2019;322:1261-70. PMID: 31573637
- Fowler AA III, Syed AA, Knowlson S, et al. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J Transl Med 2014;12:32. PMID: 24484547
- Burke JF, Sussman JB, Kent DM, Hayward RA. Three simple rules to ensure reasonably credible subgroup analyses. BMJ. 2015;351:h5651. PMID: 26537915
Guest Post By:

Wilson Smith, MD
PGY-3, Emergency Medicine Resident
Vassar Brothers Hospital, Poughkeepsie, New York
Twitter: @WTSmithMD

Marco Propersi, DO FAAEM
Vice-Chair, Emergency Medicine
Vassar Brothers Hospital, Poughkeepsie, New York
Twitter: @marco_propersi
Post-Peer Reviewed By: Salim R. Rezaie, MD (Twitter: @srrezaie)
The post The LOVIT Trial: Orange Crushed appeared first on REBEL EM - Emergency Medicine Blog.
