
Background: Bleeding complications are an uncommon but potentially a significant risk during central venous catheter (CV C) placement. Thrombocytopenia is associated with increased bleeding complications but there is no good-quality evidence showing that prophylactic platelet transfusions decrease that risk. Previous studies were retrospective and current society guidelines have differing thresholds (20,000 – 50,000 per cubic millimeter) for prophylactic transfusions. Despite being common practice, the necessity of routine prophylactic transfusions is now being called into question. CVC placement under ultrasound guidance has decreased overall complication rates and there is increased awareness of transfusion reactions, alloimmunization, acute lung injury, circulatory overload, as well as infection. Additionally, platelets are a scarce and expensive resource due to their short half-life and demand for platelets and other blood products is projected to further increase due to an aging population. No large, randomized study has yet determined the noninferiority of omitting prophylactic platelet transfusions in thrombocytopenic patients undergoing CVC placement.
C) placement. Thrombocytopenia is associated with increased bleeding complications but there is no good-quality evidence showing that prophylactic platelet transfusions decrease that risk. Previous studies were retrospective and current society guidelines have differing thresholds (20,000 – 50,000 per cubic millimeter) for prophylactic transfusions. Despite being common practice, the necessity of routine prophylactic transfusions is now being called into question. CVC placement under ultrasound guidance has decreased overall complication rates and there is increased awareness of transfusion reactions, alloimmunization, acute lung injury, circulatory overload, as well as infection. Additionally, platelets are a scarce and expensive resource due to their short half-life and demand for platelets and other blood products is projected to further increase due to an aging population. No large, randomized study has yet determined the noninferiority of omitting prophylactic platelet transfusions in thrombocytopenic patients undergoing CVC placement.
Paper: van Baarle FLF et al. Platelet Transfusion before CVC Placement in Patients with Thrombocytopenia. N Engl J Med. 2023. PMID: 37224197
Clinical Question: Is no platelet transfusion noninferior to prophylactic platelet transfusion in patients with thrombocytopenia (10,000 – 50,000 per cubic millimeter) undergoing ultrasound-guided central venous catheter placement in terms of catheter related bleeding risk?
What They Did:
- Unblinded, Multicenter, Randomized, Controlled, Noninferiority trial
- 10 hospitals in the Netherlands from February 2016 to March 2022
- Hematology wards and the ICU
- Thrombocytopenic (10,000-50,000 mm3) patients randomized 1:1 prior to ultrasound guided CVC placement:
- No Transfusion vs. 1 Unit Platelet Concentrate Transfusion
Outcomes:
-
Primary: Catheter-related bleeding (Grade 2 to 4)* at 1-hour and 24-hours post insertion
- No Transfusion vs. Transfusion,
- Upper margin two-sided 90% CI, relative risk of 3.5
- Non-inferiority margin was set at 2.5% in the no transfusion group
-
Secondary:
- Severe bleeding (Grade 3 to 4) within 24 hours
- Minor bleeding (Grade 1)
- Platelet or RBC transfusion after CVC placement
- Platelet/Hgb levels at 1-hour and 24-hour post placement
- Allergic transfusion reactions within 24-hours post placement
- Acute lung injury within 48-hours post placement
- Length of stay
- In-hospital mortality
- Financial cost
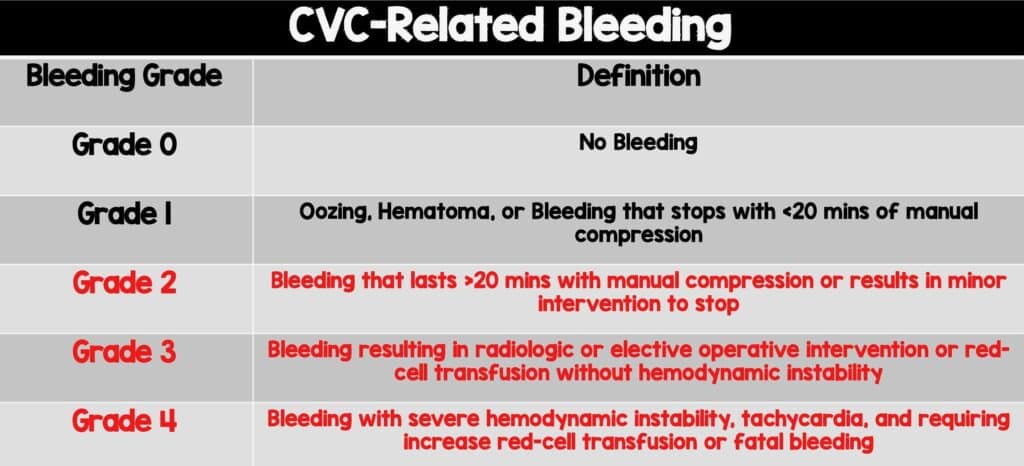 * Bleeding severity grading scale
* Bleeding severity grading scale
Inclusion:
- Thrombocytopenia 10,000 to 50,000 mm3 within 24 hours prior to procedure
- CVC required to be in place 24 hours after procedure
Exclusion:
- Therapeutic anticoagulation
- Congenital or acquired coagulation factor deficiency or bleeding risk
- Prolonged INR > 1.5 (later adjusted to INR > 3.0)
- Multiple placement episodes on patients allowed but not within 24 hours after prior randomization
Results:
- 393 CVC placements involving 358 patients included in intention-to-treat analysis
- 20 patients excluded due to protocol violations
- Transfusion (n = 188) vs. No Transfusion (n = 185)
-
Well balanced patient characteristics
- Transfusion vs No Transfusion
- Median age 58 vs. 59 years
- Median platelet count 30,000 mm3
- Median INR 1.1
- Median hemoglobin: 8.2 g/dl vs 8.5 g/dl
-
Location:
- Hematology ward 57.4 vs. 56.2%
- ICU: 42.6% vs 43.8%
-
Catheter Type:
- CVC: 82.4% vs 83.8%
- Dialysis: 17.6% vs 16.2%
-
Catheter Site:
- Internal jugular 49.5 vs. 50.3%
- Subclavian: 37.8% vs 37.8%
- Femoral: 12.8% vs 11.9%
- Transfusion vs No Transfusion
-
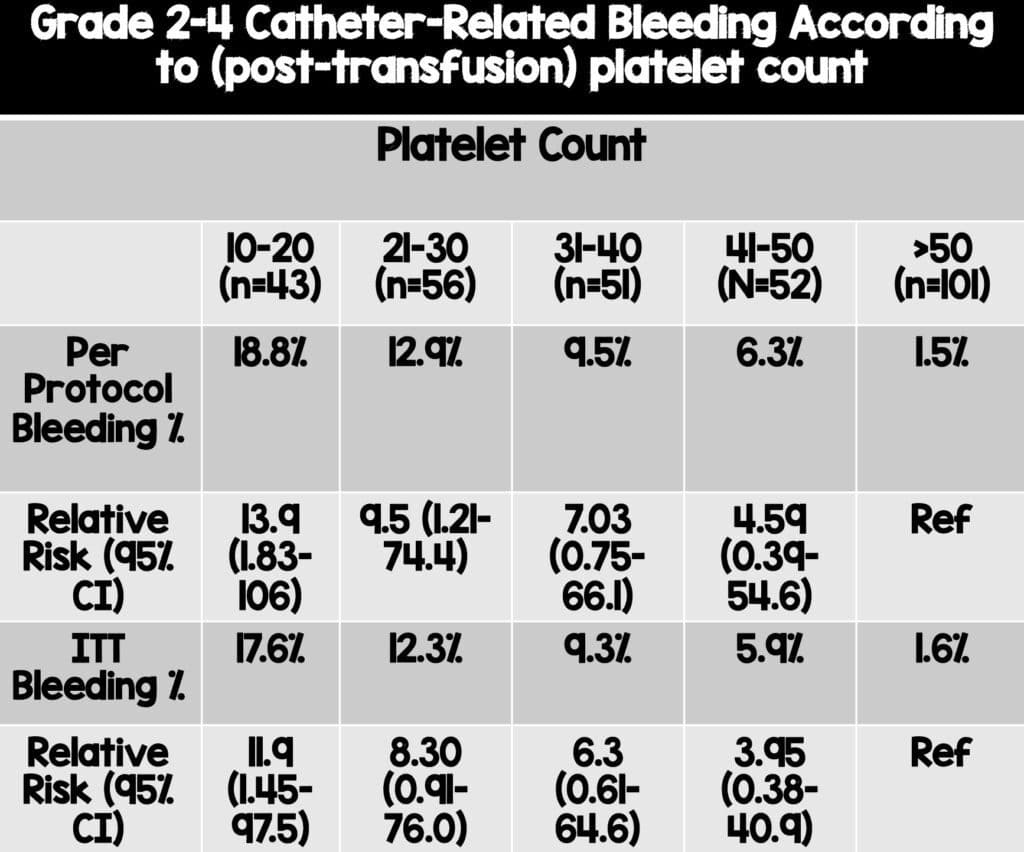
Primary Outcome – Bleeding (Grade 2 to 4)
- Transfusion 4.8% vs No Transfusion 11.9%
- Noninferiority of the no transfusion strategy was not shown
- Transfusion group had a 7.1% absolute risk difference (90% CI 1.3-17.8) and a relative risk reduction of 2.45 (90% CI: 1.27-4.70) in grade 2-4 bleeding event
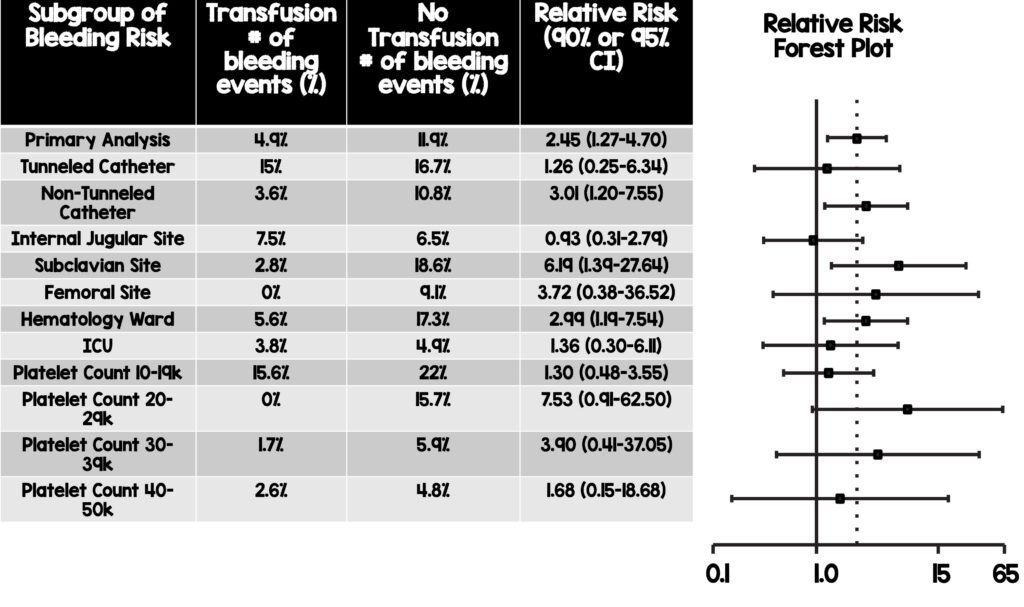
- Subgroup analysis revealed a higher risk of grade 2-4 bleeding was noted with decreasing platelet counts, and the greatest benefit of platelet transfusion is likely to be in those patients with a lower platelet count.
-
Key Secondary Outcome – Severe Bleeding (Grade 3 to 4)
- Transfusion 2.1% vs No Transfusion 4.9%
- RR 2.43 (95% CI: 0.75 – 7.93)
- No Grade 4 bleeding complications in either group
-
Other Selected Secondary Outcomes (Transfusion vs No Transfusion)
- Platelet count after CVC placement
- 1 Hour: 54,000 mm3 vs 26,000 mm3; 24 Hours: 36,000mm3 vs 26,000mm3
- Hematoma Occurrence: 12.2% vs 18.9%
- Rate of Platelet Transfusion within 24 hours: 0.14 vs. 0.47
- Rate of Red-Cell Transfusion within 24 hours: 0.48 vs 0.49
- Allergic Transfusion Reactions: 1% vs. 0.5%
- Acute Lung Injury: 0.5% vs 0%
- ICU length-of-stay: 9 days vs 7 days
- In-Hospital Mortality: 28.2% vs 31.7%
- ICU Mortality: 56.7% vs 51.8%
- Transfusions increased cost per CVC placement by $410 (95% CI: 285 to 545)
- Platelet count after CVC placement
Strengths:
- Muti-center, randomized controlled trial
- Noninferior trial design and relevant primary outcome related to safety
- Adds further information to a question with inconsistent previous research and consensus
- Broad inclusion criteria reflect common practice
- Adequate sample size relative to event rate, multicenter recruitment, standardized bleeding scale and randomized trial design improve generalizability of results
- No loss to follow-up
- Cost analysis
Limitations:
- Limited to study centers and healthcare system in the Netherlands which limits external validity and generalizability to lower income countries
- Unblinded study where the operator was unaware of trial assignment but not patient or care team which could lead to Hawthorne effect where the care team was more careful when aware of the group assignments, potentially impacting bleeding rates.
- One unit platelet transfusion strategy regardless of what the initial platelet count was and no recheck of platelet count post transfusion. This may call into question whether 1 unit of platelets is sufficient for certain patients, especially those with a lower platelet count (10,000-20,000mm3).
- Authors note that compared to previously published data, the overall incidence of bleeding was markedly higher in this trial but note that this is likely due to the prospective and structured manner of bleeding assessment in this RCT as opposed to previous evidence being based on retrospective data.
Discussion:
- A strategy of no prophylactic platelet transfusions was not found to be noninferior to routine prophylactic transfusions in thrombocytopenic patients undergoing central catheter placement.
- Patients on the hematology wards, receiving tunneled catheters, as well as those with platelet count of 10,000-20,000mm3 had the highest incidence of bleeding in this study and should be strongly considered for prophylactic transfusions.
- Subgroup analysis also revealed that although not statistically significant, there was a trend towards less bleeding in those receiving prophylactic transfusions in the subclavian (2.8% vs 18.6%) and femoral sites (0% vs 9.1%) as well as a trend towards less bleeding for patients in the hematology ward receiving prophylactic transfusions (5.6% vs 17.3%). This patient population and the sites of CVC insertion may warrant particular attention when considering prophylactic transfusion.
- Bleeding complications when measured in a trial setting may be more common than previously thought. Noninferior margins were based on a predicted primary outcome event rate of 1% (vs. actual event rate 4.8%) in the transfusion group.
- The no transfusion strategy was associated with an upfront savings of $410. Although associated with an overall cost reduction, the majority of patients in the no transfusion group with a platelet count of 10,000-30,000mm3 still received a platelet transfusion within 24 hours after CVC placement, especially on the hematology ward, which mitigates some benefits of the conservative transfusion approach.
- Rates of transfusion reactions were low.
Author Conclusion: “In patients with severe thrombocytopenia, we found that withholding prophylactic platelet transfusion before CVC placement in those with a platelet count of 10,000 to 50,000 per cubic millimeter did not meet the predefined margin for noninferiority and resulted in more CVC-related bleeding than prophylactic platelet transfusion.”
Clinical Take Home Point: In patients with severe thrombocytopenia (10,000-50,000 mm3) receiving ultrasound guided CVC, withholding prophylactic platelet transfusion prior to CVC placement did not meet the predefined margin for non-inferiority and resulted in more CVC-related bleeding than prophylactic platelet transfusion. However, a more personalized approach should be considered where prophylactic platelets should be strongly considered in patients with a platelet count <30,000mm3, those on hematology wards, as well as those undergoing tunneled catheter placement. Conversely, it may be reasonable to withhold prophylactic platelets for patients in the ICU setting due to trends of lower bleeding risk noted as well as more intensive bleeding monitoring. Future trials should follow up to see if there are benefits to transfusion of multiple units of platelets in those with a platelet count < 20,000mm3 due to their bleeding risk remaining high even after transfusion of one unit of platelets in this trial.
References:
- van Baarle FLF et al. Platelet Transfusion before CVC Placement in Patients with Thrombocytopenia. N Engl J Med. 2023. PMID: 37224197
Guest Post By:

Clifford Chang, MD
PGY-2 Emergency Medicine Resident
Inspira Medical Center, Vineland, NJ

Muhammad Durrani, DO
Assistant Clerkship Director, Assistant Research Director
Inspira Medical Center, Vineland, NJ
Post Peer Reviewed By: Salim R. Rezaie, MD (Twitter/X: @srrezaie)
The post The PACER Trial: Platelet Transfusion before CVC Placement in Patients with Thrombocytopenia appeared first on REBEL EM - Emergency Medicine Blog.
