
 Background: Hyponatremia is one of the most common electrolyte abnormalities seen in clinical practice. Under-correction could lead to cerebral edema, whereas overcorrection could result in osmotic demyelination syndrome (ODS). The current recommendation is to use hypertonic saline in small, fixed, intermittent boluses. This approach avoids rapid partial correction of serum sodium, limits risk of overcorrection, and doesn’t require complex calculations. Slow continuous infusions on the other hand, require complex calculations to adjust hypertonic saline infusions to a rate of correction over time based on the rate of serum sodium correction. Despite these facts, there are limited high-quality trials that have demonstrated if slow continuous infusion (SCI) therapy is as good as or as safe as rapid intermittent bolus (RIB) therapy.
Background: Hyponatremia is one of the most common electrolyte abnormalities seen in clinical practice. Under-correction could lead to cerebral edema, whereas overcorrection could result in osmotic demyelination syndrome (ODS). The current recommendation is to use hypertonic saline in small, fixed, intermittent boluses. This approach avoids rapid partial correction of serum sodium, limits risk of overcorrection, and doesn’t require complex calculations. Slow continuous infusions on the other hand, require complex calculations to adjust hypertonic saline infusions to a rate of correction over time based on the rate of serum sodium correction. Despite these facts, there are limited high-quality trials that have demonstrated if slow continuous infusion (SCI) therapy is as good as or as safe as rapid intermittent bolus (RIB) therapy.
Paper: Baek SH et al. Risk of Overcorrection in Rapid Intermittent Bolus vs Slow Continuous Infusion Therapies of Hypertonic Saline for Patients with Symptomatic Hyponatremia: The SALSA Randomized Clinical Trial. JAMA Intern Med 2021. PMID: 33104189 [Access on Read by QxMD]
Clinical Question: What is the risk of overcorrection with hypertonic saline (3%) in rapid intermittent bolus (RIB) vs slow continuous infusion (SCI) therapies in patients with symptomatic hyponatremia?
What They Did:
- Prospective, multicenter, open label, randomized clinical trial performed in the Republic of Korea
- Hypertonic saline (3%) given for 24 to 48hrs
- Serum sodium measured every 6hrs for 2 days
- Treatment based on symptom severity and patients randomized to:
RIB Treatment
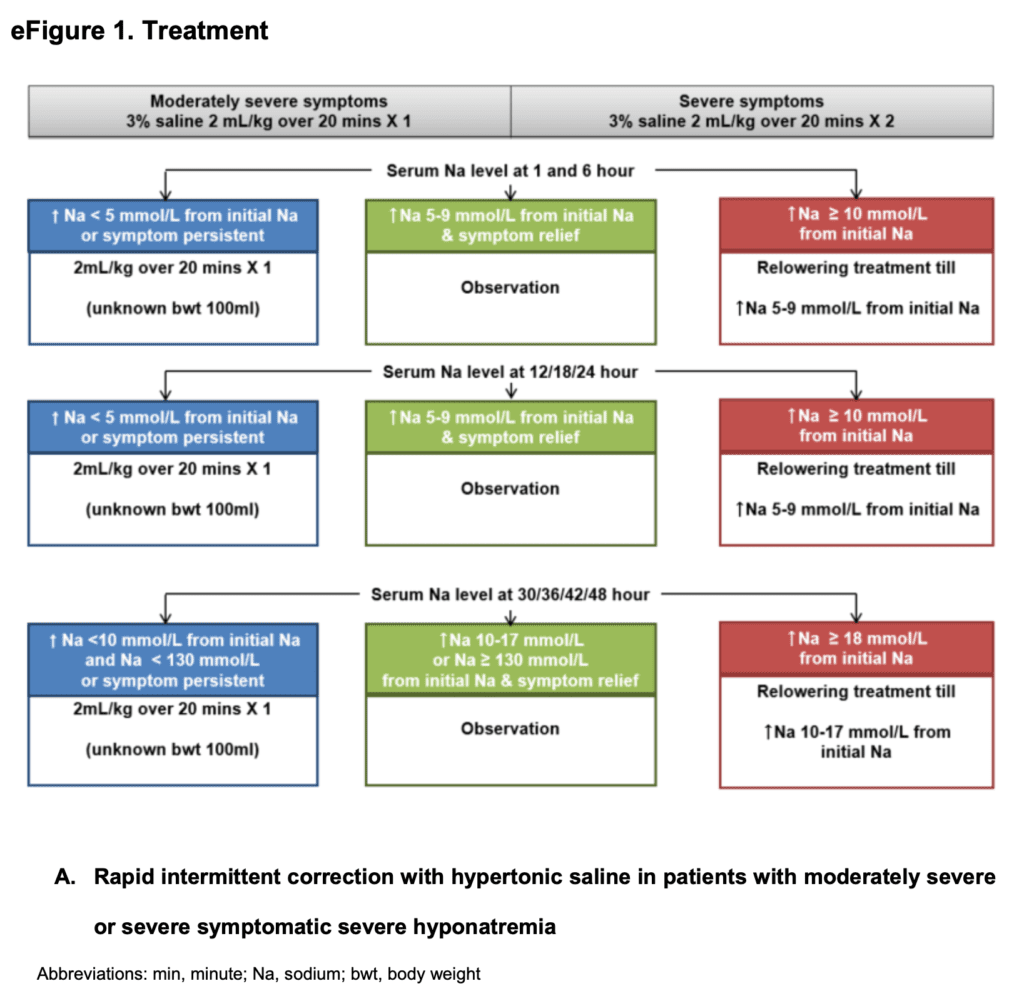
SCI Treatment
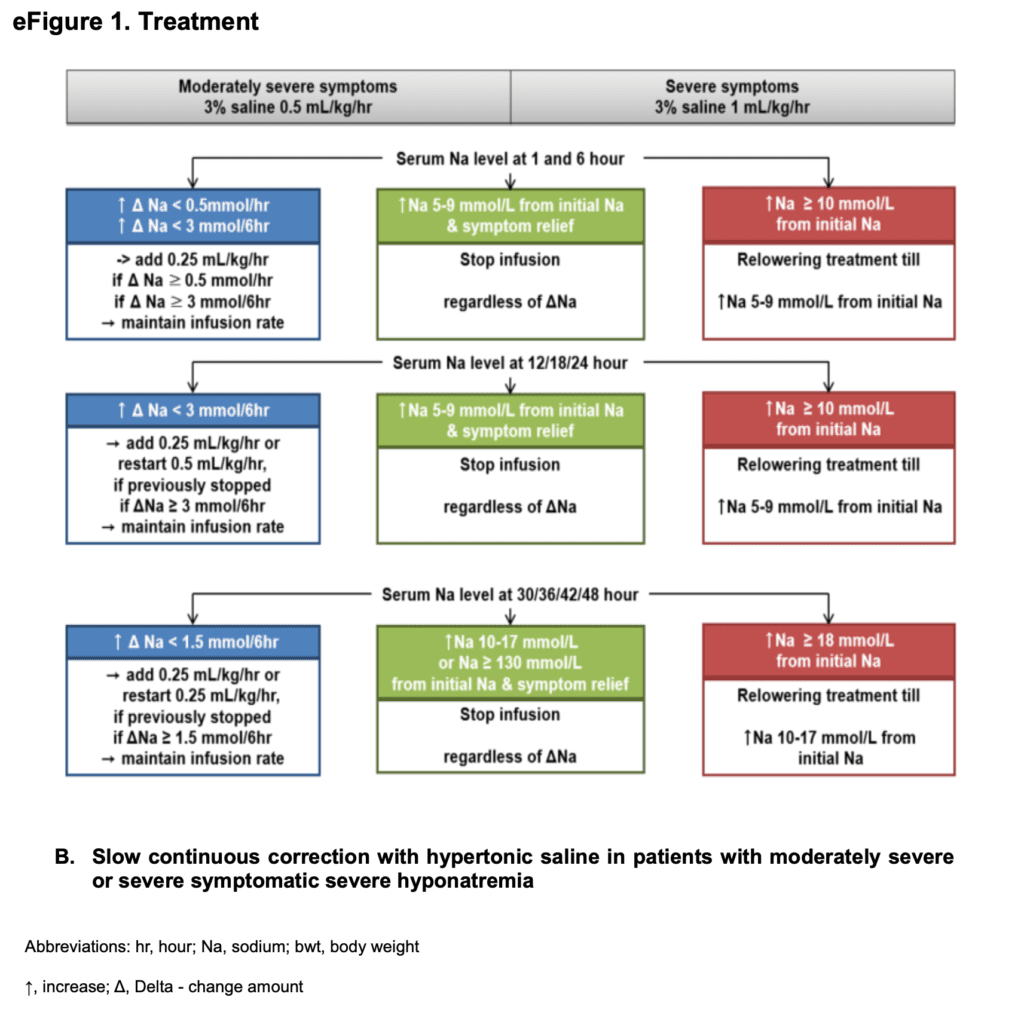
Outcomes:
-
Primary: Incidence of overcorrection at any given period
- Defined as increase in serum sodium level by greater than 12 mmol/L within 24 hours or 18mmol/L 48 hours
-
Secondary:
- Efficacy and safety of treatment approaches
- Whether symptoms remained at 24 and 48hrs after tx with hypertonic saline
- First time to an increase in serum sodium of ≥5mmol/L after treatment initiation
- Time from treatment initiation to achievement of serum sodium >130mmol/L
- Incidence of target correction rate (defined as achieving serum sodium 5 to 9mmol/L within 24hrs and serum sodium of 10 to 17 mmol/L or ≥130mmol/L within 48hrs)
- Hospital LOS
- Incidence of additional treatment
- Incidence of ODS
- Change in GCS between pretreatment and 2r and 48hrs after treatment
Inclusion:
- Patients ≥18 years of age
- Moderately severe to severe symptoms
- Moderate symptoms defined as nausea, headache, drowsiness, general weakness, and malaise)
- Severe symptoms defined as vomiting, stupor, seizure, and coma (GCS ≤8)
- Glucose corrected serum sodium of ≤125mmol/L
Exclusion:
- Primary polydipsia (urine osmolality ≤100mOsm/kg)
- Pregnant or breast feeding
- Anuria
- Arterial hypotension (SBP <90mmHg)
- MAP < 70mmHg
- Liver disease (transaminase levels >3x the ULN, known decompensated liver cirrhosis with ascites or diuretic use, hepatic encephalopathy, and varices)
- Uncontrolled DM (HbA1c >9%)
- History of cardiac surgery, MI, sustained VT/VF, ACS, cerebral trauma, increased ICP within 3 months prior to randomization
- Pseudohyponatremia (serum osmolality >275mOsm/kg) HOWEVER if serum osmolality >275mOsm/kg but ≥BUN 30mg/dL pts included if calculated serum osmolality <275mOsm/kg
Results:
- 178 patients randomized
- Mean age = 73.1 years
- Mean sNa = 118.2mmol/L
- Most Common Causes of Hyponatremia:
- Thiazide diuretics: 29.8%
- SIADH: 29.2%
- Adrenal Insufficiency: 16.3%
- Hypertonic Saline Initiated in the ED: 73.6% of cases
- 175pts (98.3%) received hypertonic saline through a peripheral IV
-
Overcorrection of Sodium (ITT Population):
- RIB: 17.2%
- SCI: 24.2%
- Absolute risk difference: -6.9%; 95% CI -18.8% to 4.9%; p = 0.26
- Additional Treatment Provided:
- RIB: 90.8%
- SCI: 74.7%
- Absolute Risk Difference: 16.1%; 95% CI 5.3% to 26.9%; p = 0.005
- NNT = 6.2
- Incidence of Relowering Treatment:
- RIB: 41.4%
- SCI: 57.1%
- Absolute risk difference: -15.8%; 95% CI -30.3% to -1.3%; p = 0.04
- NNT = 6.3
- No significant differences between groups observed in:
- Symptoms at 24 and 48hrs after treatment initiation
- 1st time to an increase in sNa ≥5mmol/L after treatment initiation
- Incidence of target correction rate
- Time from treatment initiation to achievement of sNa > 130mmol/L
- Hospital LOS
- Number of events of ODS
- No difference between groups in terms of efficacy in increasing serum sodium concentrations nor improving symptoms, BUT RIB showed better efficacy in achieving target correction rate within 1hr compared to SCI
- ITT Analysis:
- RIB: 32.2%
- SCI: 17.6%
- Absolute risk difference 14.6%; 95% CI 2% – 27.2%; p = 0.02
- NNT = 6.8
- Per Protocol Analysis:
- RIB: 29.2%
- SCI: 16.4%
- Absolute risk difference: 12.7%; 95% CI -0.8% to 26.2%; p = 0.07
- All outcomes were statistically significant for the ITT and PP analyses with the exception of achieving the target correction rate within 1hr
- ITT Analysis:
Strengths:
- Asks a clinically important question
- 1st prospective RCT comparing efficacy and safety of RIB vs SCI with hypertonic saline
- Groups had similar baseline characteristics
- Majority of patients (81%) completed the study
- Performed subgroup analyses that showed no significant heterogeneity in the effect of treatment on primary, secondary, and post hoc outcomes in predefined subgroups
Limitations:
- 95% CIs were wide for incidence of overcorrection which could reflect imprecision to the true effect. Further larger studies would be needed to confirm this
- Urine volume of patients were monitored, however the difference between water and solute diuresis was not possible due to inability to evaluate urine electrolyte and osmolality in the trial
- A significant number of patients were excluded due to protocol violations. These dropouts occurred earlier in the trial period and gradually decreased as the study went on
- None of the primary or secondary outcomes are patient centered. Used a surrogate outcome of sodium overcorrection as the primary outcome with relowering treatment whenever overcorrection occurred. There were no cases of ODS, the true clinically relevant outcome of interest
- No adjustments for any of the secondary outcomes which means some of these outcomes could be false positive findings
- Allocation sequence was not concealed from study coordinators and could have influenced recruitment
- Changes in study enrollment due to low enrollment. Included floor patients making this a heterogenous group that is being study
- Exclusion criteria are quite complicated. For example the criteria of osmolarity makes this less applicable as many of us can’t get that number rapidly
Discussion:
- There were no significant differences in the incidence of overcorrection between the 2 groups, but RIB therapy is simpler than SCI, showed a lower incidence of relowering treatment and tended to have a higher target correction rate within 1 hour compared to SCI
- Baseline sNa concentrations were similar (mean 118.2 mmol/L) between groups, but sNa concentrations at 1 hour after treatment were significantly higher in the RIB group (mean 122.0 mmol/L vs 120.1mmol/L) and the proportion of patients reaching a target correction rate within 1hr tended to be higher as well in the RIB group
- Concomitant IV fluids and oral fluid intake were at the physician’s discretion in this trial. However, the amount of concomitant treatments were not significantly different between groups and thus not likely to have affected the results
- Important point brought up by the authors is that relowering treatment adhered to more strictly in the setting of a trial rather than what actually happens in clinical practice. With less stringent protocol adherence in reality, the risk of overcorrection could be much higher than what was reported
Author Conclusion: “This randomized clinical trial found that both RIB and SIC therapies of hypertonic saline for treating hyponatremia were effective and safe, with no difference in the overcorrection risk. However, RIB had a lower incidence of therapeutic relowering treatment and tended to have a better efficacy in achieving sNa within 1 hour than SCI. RIB could be suggested as the preferred treatment of symptomatic hyponatremia, which is consistent with the current consensus guidelines.”
Clinical Take Home Point: In this small RCT, both RIB and SCI therapy were effective and safe with no statistically significant differences in overcorrection of sodium (A 7% difference wasn’t statistically significant, but this could be an issue of the study simply being underpowered). However, RIB therapy was more effective in achieving the target correction rate in the 1st hour after treatment initiation, didn’t increase the risk of overcorrection, and is user-friendly (no calculations required) compared to SCI. Until better evidence is available RIB therapy is the treatment of choice in patients with symptomatic hyponatremia.
References:
- Baek SH et al. Risk of Overcorrection in Rapid Intermittent Bolus vs Slow Continuous Infusion Therapies of Hypertonic Saline for Patients with Symptomatic Hyponatremia: The SALSA Randomized Clinical Trial. JAMA Intern Med 2021. PMID: 33104189 [Access on Read by QxMD]
For More Thoughts on This Topic Checkout:
Post Peer Reviewed By: Anand Swaminathan, MD (Twitter: @EMSwami)
The post The SALSA Trial: Symptomatic Hyponatremia and Hypertonic Saline appeared first on REBEL EM - Emergency Medicine Blog.
