
 Background: The risk of a subsequent ischemic stroke in the first few months after an acute ischemic stroke or transient ischemic attack is approximately 5 to 10%. In these patients Aspirin has been used to prevent secondary ischemia, and trials have shown a reduced risk thereof when the P2Y12 receptor blocking antiplatelet agent Clopidogrel is added. Clopidogrel, however, requires hepatic conversion to its active form through a pathway that is inefficient in 25% of white and 60% of Asian patients and efficacy is uncertain in these patients. Not dependent on metabolic activation is the direct-acting ticagrelor with similar P2Y12 receptor blocking effect. While a trial of ticagrelor alone did not show benefit over aspirin; in their sub-group analysis of patients who had received aspirin within 7 days before randomization, treatment with ticagrelor may have reduced the risk of major vascular events. This finding suggested that the effect of aspirin received before entry into the trial might have persisted for several days after treatment and that the combination of ticagrelor and aspirin may prevent subsequent strokes.
Background: The risk of a subsequent ischemic stroke in the first few months after an acute ischemic stroke or transient ischemic attack is approximately 5 to 10%. In these patients Aspirin has been used to prevent secondary ischemia, and trials have shown a reduced risk thereof when the P2Y12 receptor blocking antiplatelet agent Clopidogrel is added. Clopidogrel, however, requires hepatic conversion to its active form through a pathway that is inefficient in 25% of white and 60% of Asian patients and efficacy is uncertain in these patients. Not dependent on metabolic activation is the direct-acting ticagrelor with similar P2Y12 receptor blocking effect. While a trial of ticagrelor alone did not show benefit over aspirin; in their sub-group analysis of patients who had received aspirin within 7 days before randomization, treatment with ticagrelor may have reduced the risk of major vascular events. This finding suggested that the effect of aspirin received before entry into the trial might have persisted for several days after treatment and that the combination of ticagrelor and aspirin may prevent subsequent strokes.
Paper: Claiborne Johnston S et al. Ticagrelor and Aspirin or Aspirin Alone in Acute Ischemic Stroke or TIA. NEJM 2020. PMID 32668111
Clinical Question: Does the addition of ticagrelor to aspirin for 30 days aid in reducing the risk of subsequent stroke or death among patients with acute non-cardioembolic cerebral ischemia?
What They Did:
- Multicenter, Randomized, Double-blind, Placebo-controlled, Parallel-group Trial conducted at 414 sites in 28 countries
- Randomization occurred within 24 hours after the onset of symptoms or, in patients in whom a stroke was evident on awakening from sleep, within 24 hours from the time last known well
- Random allocation of patients to treatment with Ticagrelor + Aspirin or Placebo + Aspirin
- Ticagrelor + aspirin: Loading doses of 180mg and 300 to 325mg respectively
- a lesser loading dose of Aspirin recommended if patients had already received aspirin after symptom onset but before randomization
- subsequent maintenance doses of ticagrelor 90 mg twice daily were administered at approximately 12-hour intervals for the remainder of the 30-day treatment period
- subsequent maintenance doses of aspirin 75 to 100 mg daily were recommended
- Placebo + aspirin: Loading doses of 180mg and 300 to 325mg respectively
- a lesser loading dose of Aspirin recommended if patients had already received aspirin after symptom onset but before randomization
- subsequent maintenance doses of aspirin 75 to 100 mg daily were recommended
- After the 30-day duration of trial treatment, patients were treated according to standards of care at the discretion of the investigator and were followed for an additional 30 days, with continued collection of data on outcomes and safety events.
- Ticagrelor + aspirin: Loading doses of 180mg and 300 to 325mg respectively
Inclusion:
- Age ≥ 40 years
- Mild-to-moderate acute non-cardioembolic cerebral ischemia
- a National Institutes of Health Stroke Scale (NIHSS) score of 5 or less
- a high-risk TIA as determined according to a score of 6 or higher on the ABCD2 scale
- symptomatic intracranial or extracranial arterial stenosis (≥50% narrowing in the diameter of the lumen of an artery that could account for the TIA)
- Not undergoing thrombolysis or thrombectomy
Exclusion:
- Intravenous or intraarterial thrombolysis or mechanical thrombectomy being planned within 24 hours
- CT or MRI of the brain showing intracranial bleeding or conditions other than cerebral ischemia that could account for neurologic symptoms or contraindicate trial treatment
- use of anticoagulation or specific antiplatelet therapy other than aspirin already having been planned
- a hypersensitivity to ticagrelor or aspirin
- a history of atrial fibrillation or ventricular aneurysm
- a cardioembolic cause of the TIA or stroke was suspected
- a carotid endarterectomy that required discontinuation of the trial medication being planned within 3 days
- a known bleeding diathesis or coagulation disorder
- a history of intracerebral hemorrhage
- a gastrointestinal bleeding within the past 6 months
- major surgery within the 30 days prior
Outcomes:
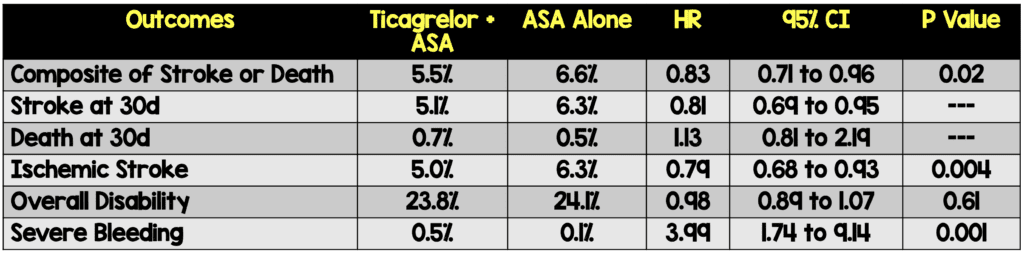
- Primary: Composite of stroke or death at 30 days
-
Secondary:
- First subsequent ischemic stroke
- Disability measured as a score of greater than 1 on the modified Rankin scale at the end of the treatment visit 30 to 34 days after randomization
-
Safety:
- Severe Bleeding defined as fatal bleeding or intracranial or other bleeding that caused hemodynamic compromise for which intervention was warranted (i.e. SBP <90mmHg that warranted blood or fluid replacement, vasopressor or inotropic support, or surgical intervention)
- Asymptomatic hemorrhagic transformation of ischemic brain infarctions and microhemorrhages smaller than 10mm were not included as severe intracranial bleeding
Results:
- 11,016 patients randomized
- Most patients (91%) presented with ischemic stoke
- 13% of patients were taking aspirin before the initial index stroke or TIA
Strengths:
- Large, Multicenter, randomized, double-blind, placebo-controlled, parallel-group trial (414 sites in 28 countries)
- Efficacy and safety analyses were based on the intention-to-treat principle and included all the patients who underwent randomization
- Baseline characteristics were similar in the two groups in terms of blood pressure, previous history, previous stroke/TIA, and NIHSS scores
- Follow up was completed in all but 15 patients in total, vital status at end of the trial was ascertained for all these patients
- Independent data and safety monitoring committee assessed conduct of the trial and a prespecified interim analysis after 70% of the targeted number of primary outcome events had occurred
Limitations:
- Large exclusion criteria, limits the population these results would apply to
- Majority of patients included had NIHSS scores of ≤3 (≈60.5%)
- 7–14.3% of patients in both groups discontinued trial treatment prematurely with most having an adverse event or severe adverse event as the cause for termination
Discussion:
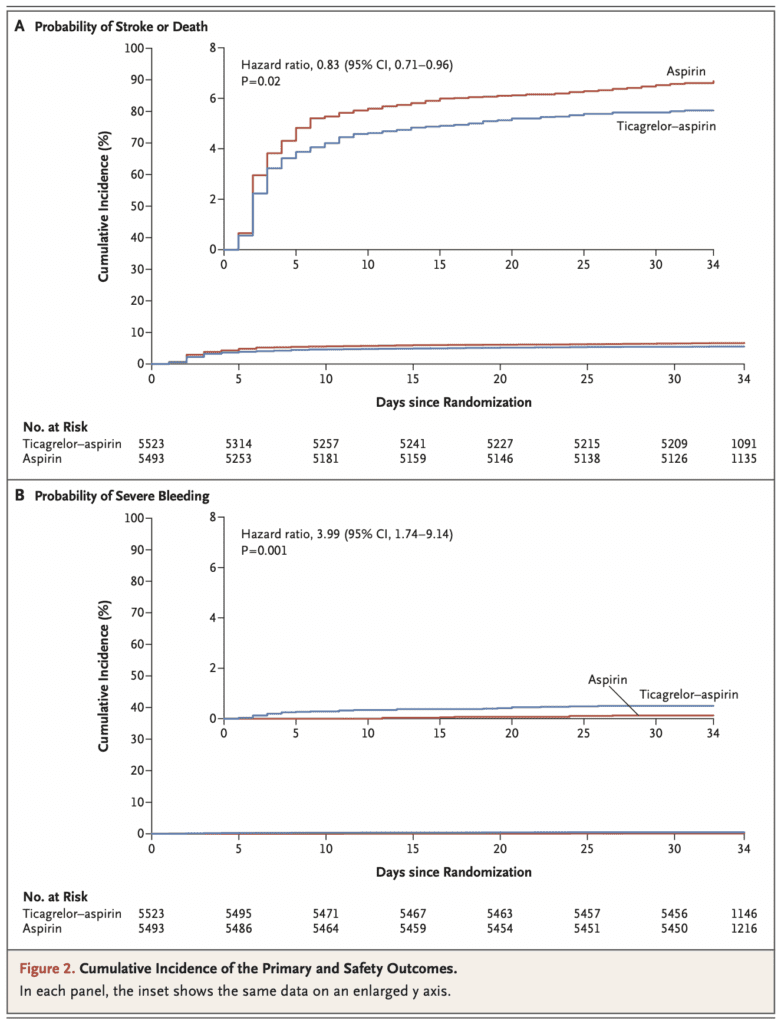
- A benefit was observed with ticagrelor + aspirin with respect to the incidence of subsequent ischemic stroke; however, without a significant effect on the incidence of overall disability
- Ticagrelor & aspirin was associated with higher risks of severe hemorrhage and cerebral hemorrhage than aspirin alone
- Number needed to treat to prevent the composite of stroke or death was 92 while the number needed to harm for severe bleeding was 263
- Only ≈20% of patients had previous stroke or TIA history
- Only 13% of patients were taking aspirin before the initial index stroke or TIA
- The results of this trial are similar to prior evidence:
- In the POINT trial, which involved an international population of patients who received 90-day treatment within 12 hours after an acute ischemic stroke or TIA, the incidence of major ischemic events (ischemic stroke, myocardial infarction, or death from an ischemic vascular event) was 5.0% among patients who received Clopidogrel & Aspirin and 6.5% among those who received Aspirin alone.
- In the CHANCE trial, the risk of stroke recurrence among Chinese patients who were treated within 24 hours after a minor ischemic stroke or TIA was 8.2% among those who received a Clopidogrel-based regimen (Clopidogrel & Aspirin for 21 days, followed by Clopidogrel alone through day 90) and 11.7% among those who received Aspirin alone.
- Differences in patient populations and outcome definitions prevent comparisons of the results of these trials with those of the current THALES trial. Generalizability of the results of the current trial is limited by the exclusion of patients who had more severe strokes (NIHSS score >5), had a cardioembolic stroke, or had initiation of treatment more than 24 hours after symptom onset or who underwent or planned to undergo thrombolysis or thrombectomy.
- In the THALES trial, similar to the POINT trial, an absolute increase in the risk of severe hemorrhage was observed, although there were a small number of events. At variance with the observations in the THALES and POINT trials, no increase in the incidence of moderate-to-severe hemorrhage was reported in the CHANCE trial.
Author Conclusion: “Among patients with mild-to-moderate ischemic stroke (NIHSS score ≤5) or TIA who were not undergoing intravenous or endovascular thrombolysis, the risk of the composite of stroke or death within 30 days was lower with ticagrelor-aspirin than with aspirin alone, but the incidence of disability did not differ significantly between the two groups. Severe Bleeding was more frequent with ticagrelor.”
Clinical Take Home Point: A benefit was observed with ticagrelor + aspirin with respect to the incidence of the secondary outcome of subsequent ischemic stroke, which was lower than with aspirin alone; however, a benefit was not observed with respect to the incidence of overall disability, and additionally there was increase in severe bleeding and intracranial hemorrhage.
Guest Post By:

Benjamin M. Gerretsen, MD
Emergency Medicine
Erasmus University Medical Center
Leiden, Netherlands
Twitter: @bmgerretsen

Barbra Backus, MD
Emergency Physician
Erasmus University Medical Center
Netherlands
Twitter: @barbrabackus
References:
- Claiborne Johnston S et al. Ticagrelor and Aspirin or Aspirin Alone in Acute Ischemic Stroke or TIA. NEJM 2020. PMID 32668111
- Claiborne Johnston S et al. Platelet-oriented inhibition in new TIA and minor ischemic stroke (POINT) trial. Int J Stroke 2013. PMID: 23879752
- Wang Y et al. Clopidogrel With Aspirin in Acute Minor Stroke or Transient Ischemic Attack (CHANCE) trial. Circulation 2015. PMID: 25957224
- Claiborne Johnston S et al. The Acute STroke or Transient IscHemic Attack Treated with TicAgreLor and Aspirin for PrEvention of Stroke and Death (THALES) trial. Int J Stroke 2019. PMID: 30747613
For More Thoughts on This Topic Checkout:
Post Peer Reviewed By: Salim R. Rezaie, MD (Twitter: @srrezaie)
The post The THALES Trial: Ticagrelor and Aspirin vs Aspirin Alone in Acute Ischemic Stroke or TIA appeared first on REBEL EM - Emergency Medicine Blog.
