
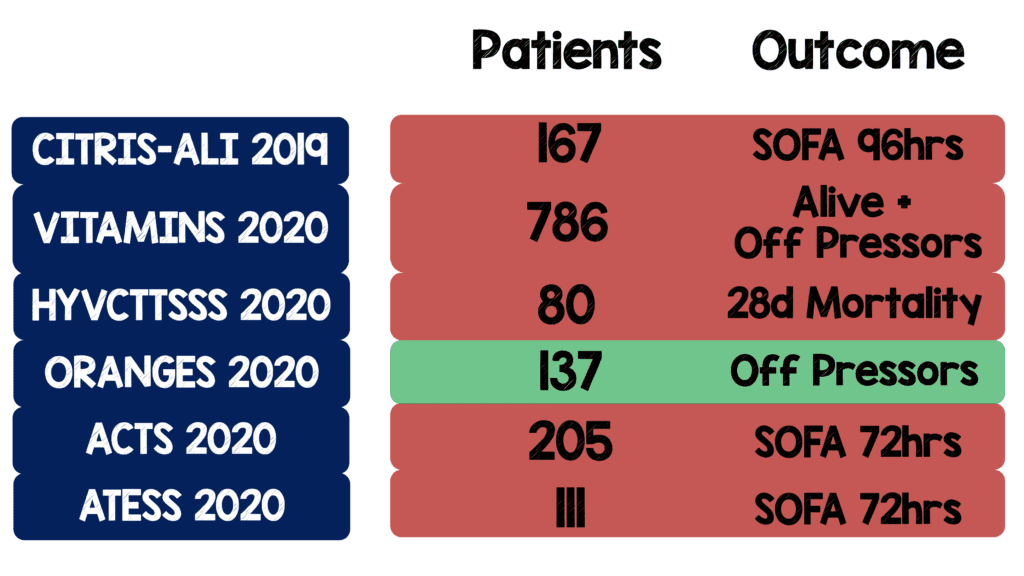
 Background: Since the publication of the before and after Marik trial [1] published in 2016, there have been six randomized clinical trials trying to answer the question of the utility of the metabolic cocktail (Vitamin C, thiamine, and hydrocortisone) in septic shock (See table below). Although, each was of various methodological rigor only one had a positive primary outcome (i.e. ORANGES). It is important to note that the primary outcome of the ORANGES trial was changed after full data collection (and likely analysis) was complete (See the REBEL EM analysis HERE). We now have our 7th RCT on this topic.
Background: Since the publication of the before and after Marik trial [1] published in 2016, there have been six randomized clinical trials trying to answer the question of the utility of the metabolic cocktail (Vitamin C, thiamine, and hydrocortisone) in septic shock (See table below). Although, each was of various methodological rigor only one had a positive primary outcome (i.e. ORANGES). It is important to note that the primary outcome of the ORANGES trial was changed after full data collection (and likely analysis) was complete (See the REBEL EM analysis HERE). We now have our 7th RCT on this topic.
Paper: Sevransky JE et al. Effect of Vitamin C, Thiamine, and Hydrocortisone on Ventilator- and Vasopressor-Free Days in Patients with Sepsis: The VICTAS Randomized Clinical Trial. JAMA 2021. PMID: 33620405
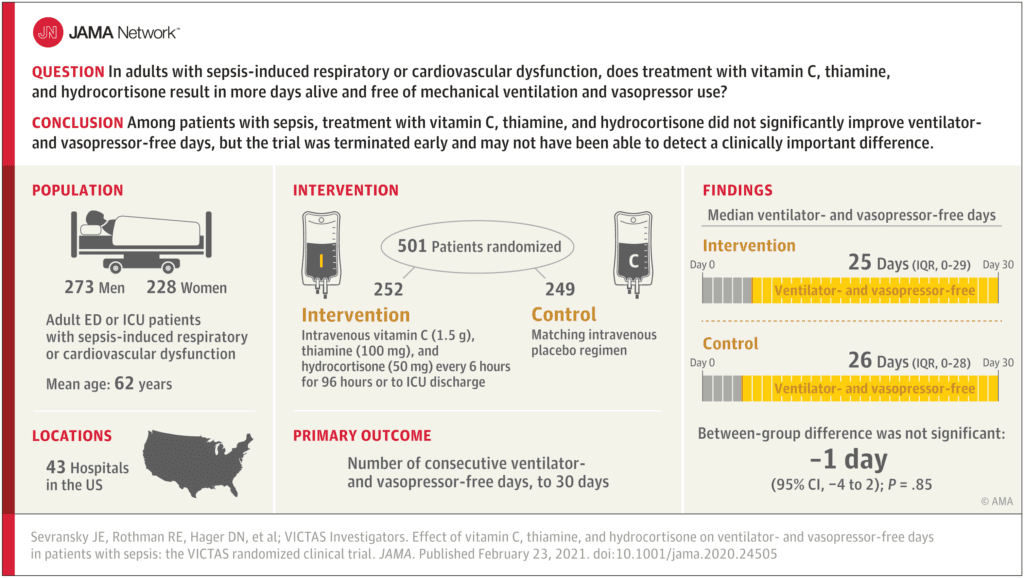
Clinical Question: In adults with sepsis-induced respiratory or cardiovascular dysfunction, does treatment with vitamin C, hydrocortisone and thiamine result in an increase in the number of days alive and free of mechanical ventilation and vasopressor use?
What They Did:
- The Vitamin C, Thiamine, and Steroids in Sepsis (VICTAS) Trial
- Multicenter, randomized, double-blind, adaptive-sample-size, placebo-controlled trial
- Patients enrolled in EDs or ICUs at 43 hospitals in the US
- After enrollment of 501 patients, funding was withheld, leading to an administrative termination of the trial
- Patient were randomized within 24 hours of organ dysfunction to:
- Metabolic Cocktail IV q6hrs x96hours or until discharge from the ICU or death (Same protocol as the Marik publication):
- Vitamin C 1.5g
- Thiamine 100mg
- Hydrocortisone 50mg
- Matching Placebo
- Metabolic Cocktail IV q6hrs x96hours or until discharge from the ICU or death (Same protocol as the Marik publication):
Outcomes:
- Primary: Median number of consecutive ventilator- and vasopressor-free days in the first 30d following randomization
-
Secondary (There were 18 secondary outcomes):
- 30d Mortality
Inclusion:
- ≥18 years of age
- Acute respiratory and/or cardiovascular dysfunction caused by suspected infection
- Planned ICU admission
Exclusion: None
Results:
- 501 patients randomized
- Median APACHE II score = 27
- Median SOFA Score = 9
- 41% required both ventilatory and vasopressor support
- 21% required ventilatory support alone
- 38% required vasopressor support alone
- Open-label corticosteroids were prescribed in ≈33% of patients in both groups
- Median time from onset of organ dysfunction and first dose of study drug = 14.7hrs
- Median Ventilator- and Vasopressor-Free Days:
- Metabolic Cocktail: 25d
- Placebo: 26d
- Difference of -1d; 95% CI -4 to 2d; p = 0.85
- 30d Mortality:
- Metabolic Cocktail: 22%
- Placebo: 24%
- No difference in mortality at 180d, change in median SOFA score, length of ICU stay, renal replacement therapy free days
- No serious adverse events in either group
Strengths:
- Severity of illness, comorbidities, and source of infection were similar between groups
- High protocol adherence with intervention and placebo
- Randomization and blinding adequately performed
- Used the same cocktail as the Marik trial
- Multicenter trial increasing external validity
- Asks an important question
- High adherence to protocol
- Performed intention to treat and per-protocol analysis
Limitations:
- Primary outcome composed of two pieces that are not equal in relevance to patient
- Death/disability would have been a more objective primary outcome
- Appears to be a convenience sample
- Did not break down primary outcome into components to see if one was the primary driver
- Trial was designed to detect a difference in the primary outcome of 1.5 days with recruitment of up to 2000 patients which was based on a mortality rate of 25% which was not achieved in the study
- Trial was stopped early after ¼ of patients recruited
- Trial underpowered to detect a difference
Discussion:
- Reviewing the primary outcomes of the previous 6 RCTs here are the results:
- Below is a table I created summarizing all the RCTs with timing of intervention, mortality, and time of vasopressors:
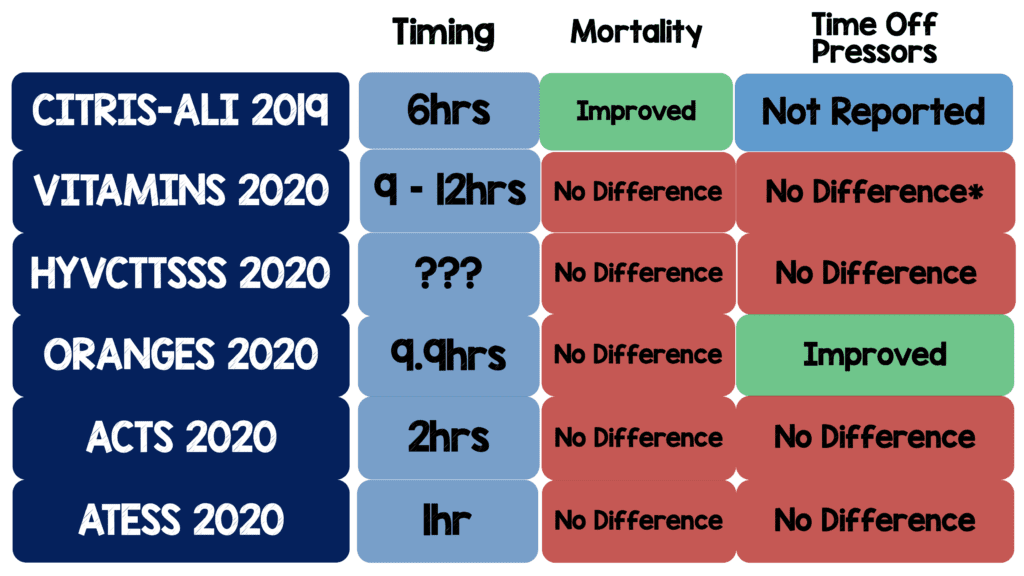
- It’s worth mentioning that CITRIS-ALI is the only RCT to show a reduction in mortality however there are some nuances to this trial:
- Mortality was one of 46 secondary outcomes. If you have 46 secondary outcomes, it is likely some will be positive by random chance alone and therefore statistical noise
- The dosing of vitamin C was much higher than other trials (50mg/kg q6hrs) as well as focusing on patients with acute lung injury
- The ORANGES trial is the only RCT to show an improvement in time off vasopressors BUT…
- According to clinicaltrials.gov the study was submitted on January 30th, 2018 and on June 11th, 2019 the primary outcome was changed from in-hospital morality to time to vasopressor independence. This change was made after all the data was collected. It is unclear if the authors had analyzed the data or not, however it is suspicious when a change is made this late in the process. The original primary outcome (in-hospital mortality) was not statistically significant making this change even more suspect.
Author Conclusion: “Among critically ill patients with sepsis, treatment with vitamin C, thiamine, and hydrocortisone, compared with placebo, did not significantly increase ventilator- and vasopressor-free days within 30 days. However, the trial was terminated early for administrative reasons and may have been underpowered to detect a clinically important difference.”
Clinical Take Home Point: This is now the 7th RCT looking at the use of the metabolic cocktail (vitamin C, thiamine, and hydrocortisone) in the management of sepsis/septic shock. Throwing more research resources behind an initiative that is not supported by evidence is a distraction when the resources available are finite. This issue should have been settled after the first 3 RCTs, there is no point in continuing to beat on a dead horse.
References:
- Marik PE et al. Hydrocortisone, Vitamin C, and Thiamine for the Treatment of Severe Sepsis and Septic Shock: A Retrospective Before-After Study. CHEST 2017. PMID: 27940189
- Sevransky JE et al. Effect of Vitamin C, Thiamine, and Hydrocortisone on Ventilator- and Vasopressor-Free Days in Patients with Sepsis: The VICTAS Randomized Clinical Trial. JAMA 2021. PMID: 33620405
- Walter KL et al. Hydrocortisone, Vitamin C, and Thiamine for Treatment of Sepsis: Making Evidence Matter. JAMA 2021. PMID: 33620384
Post Peer Reviewed By: Anand Swaminathan, MD (Twitter: @EMSwami)
The post The VICTAS Trial: How Many More RCTs do we Need on the Metabolic Cocktail in Sepsis? appeared first on REBEL EM - Emergency Medicine Blog.
