
 Background Information: Nausea and vomiting during pregnancy most commonly occurs during the first trimester. If left untreated, the development of hyperemesis gravidarum can lead to further complications characterized by dehydration and electrolyte abnormalities.1 Ondansetron, a 5-HT3 receptor antagonist has quickly become the most frequently prescribed drug in the United States for nausea and vomiting during pregnancy.2 With the creation of an oral dissolving tablet in 2006, Ondansetron’s popularity as an antiemetic continues to rise. In fact, a study from 2014 shows that nearly a quarter of all pregnant women in the United States are using it.3 There is uncertainty in the literature as to the association between Ondansetron and birth defects. While some studies report there is no increased risk in congenital abnormalities among women who took this antiemetic early in pregnancy, other evidence suggests it may be associated with cleft palate and cardiac malformations.2 The authors of this study sought to investigate the association between exposure to Ondansetron during the first trimester of pregnancy and risk of congenital malformations in newborns using a national cohort of publicly insured pregnant women.
Background Information: Nausea and vomiting during pregnancy most commonly occurs during the first trimester. If left untreated, the development of hyperemesis gravidarum can lead to further complications characterized by dehydration and electrolyte abnormalities.1 Ondansetron, a 5-HT3 receptor antagonist has quickly become the most frequently prescribed drug in the United States for nausea and vomiting during pregnancy.2 With the creation of an oral dissolving tablet in 2006, Ondansetron’s popularity as an antiemetic continues to rise. In fact, a study from 2014 shows that nearly a quarter of all pregnant women in the United States are using it.3 There is uncertainty in the literature as to the association between Ondansetron and birth defects. While some studies report there is no increased risk in congenital abnormalities among women who took this antiemetic early in pregnancy, other evidence suggests it may be associated with cleft palate and cardiac malformations.2 The authors of this study sought to investigate the association between exposure to Ondansetron during the first trimester of pregnancy and risk of congenital malformations in newborns using a national cohort of publicly insured pregnant women.
Paper: Huybrechts KF, et al. Association of Maternal First-Trimester Ondansetron Use with Cardiac Malformations and Oral Clefts in Offspring. JAMA. 2018. PMID: 30561479
Clinical Question: Is exposure to ondansetron during the first trimester of pregnancy associated with an increased risk of congenital malformations in newborns (focusing on cardiac malformations and oral clefts)?
What They Did:
- A retrospective cohort study looking at ≈1.8 million pregnancies from the nationwide Medicaid Analytic eXtract (MAX) which has been previously used to study the safety of medications during pregnancy.4
- Women were considered “exposed” if they filled at least one Ondansetron prescription during the first 3 months of pregnancy (period of organogenesis).
- Women were considered “unexposed” if they did not fill at least one Ondansetron prescription during the first 3 months of pregnancy
- Women who filled a prescription during the first 90d of pregnancy for pyridoxine (with or without doxylamine), promethazine, metoclopramide, or any of these alternative treatments were used as an alternative reference group
- Presence of congenital malformations was defined using an algorithm based on inpatient or outpatient diagnoses and procedural codes in the maternal or fetal record
- The authors also looked at potential confounders and/or proxies for confounders
- The authors used propensity scores to adjust for a large number of covariates and the following analyses were conducted:
- Primary adjusted analysis = To account for possible confounding effect of the indication for treatment and its associated factors
- Secondary adjusted analysis = To account for all potential confounding variables. These results were interpreted as exploratory.
- Confirmatory analysis = High dimensional propensity score analysis included 200 empirically defined covariates in addition to the prespecified covariates.
- Sensitivity analyses = Conducted to test the robustness of the primary results
- The two groups (exposed and unexposed) were assessed using standardized differences. An absolute standardized difference greater than 0.1 was considered an indicator for substantial imbalances between the two exposure groups.
- Absolute, unadjusted relative risks and risk differences with their 95% confidence intervals were calculated for each of the outcomes
Inclusion Criteria:
- Women aged 12 to 55 years old
- Medicaid coverage from 3 months before the date of their last menstrual period to 1 month after delivery
- Filled at least one Ondansetron prescription during the first three months of pregnancy
- Infants with Medicaid coverage for the first three months of life, unless they died sooner
Exclusion Criteria:
- Pregnancies with exposure to other known teratogens (i.e. Warfarin, Antineoplastic agents, Lithium, Isotretinoin, Misoprostol, Thalidomide, etc.)
- Pregnancies with a chromosomal abnormality
- Women who filled an Ondansetron prescription in the 3 months before pregnancy
Outcomes:
- Primary
-
- Cardiac malformations diagnosed during 1st 90d after delivery
- Oral cleft malformations diagnosed during 1st 90d after delivery
- Secondary
-
- Congenital malformations overall
- Specific subgroups of cardiac malformations (i.e. Conotruncal defects, single ventricle defects, ventricular septal defects, etc.)
- Specific subgroups of oral cleft malformations (i.e. Cleft palate, Cleft lip, Cleft lip and palate)
- Risks of other specific malformations were evaluated (Eye, ear, CNS, respiratory, GI, urinary, genital, etc.)
What They Changed:
- During their analysis the authors changed the reference group to women who filled a prescription for a different antiemetic during their first trimester to be more comparable with women exposed to Ondansetron than women who were never treated with antiemetics during pregnancy
- Exposure was redefined as having filled 2 or more prescriptions for Ondansetron during the first trimester and exposure window defined as 6 to 12 weeks after the date of the last menstrual period
Results:
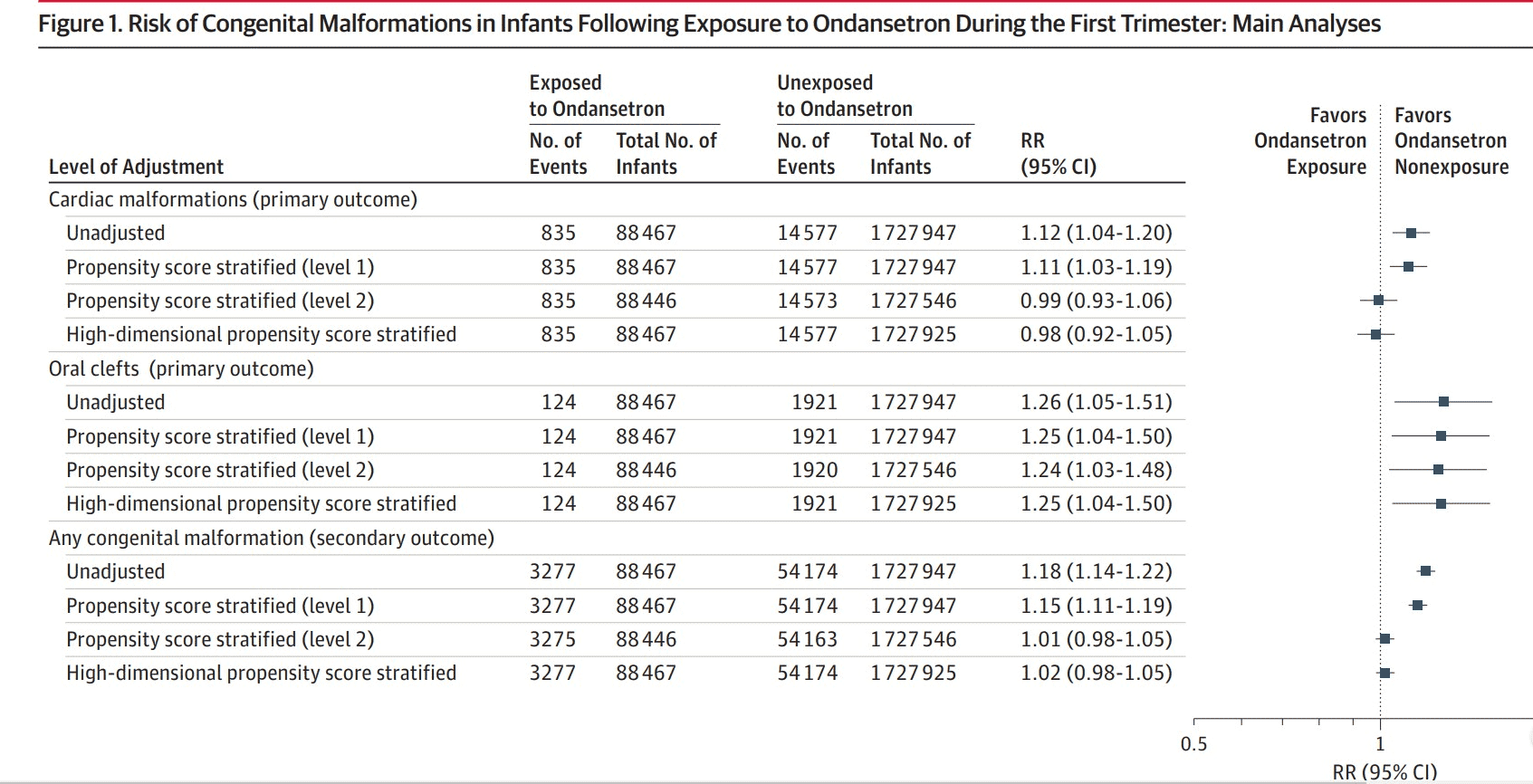
- Out of the 1,816,144 total pregnancies, 88,467 (4.9%) were exposed to Ondansetron while 1,727,947 were unexposed.
- There was a total of 1,502,895 women who contributed the following to the cohort:
- 1 pregnancy: 1,251,216 (83.3%)
- 2 pregnancies: 203,037 (13.5%)
- 3 or more pregnancies: 48, 642 (3.2%)
Critical Results:
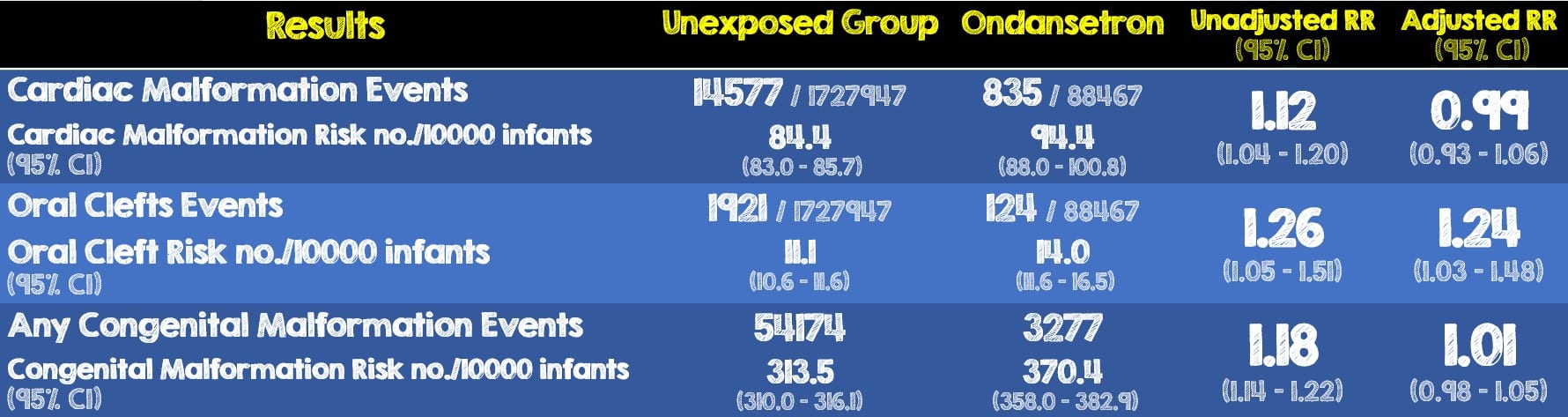
- There was a small increase in the risk of oral clefts associated with Ondansetron exposure. The upper limit of the 95% CI adjusted relative risk was 1.48 which corresponds to 5 additional cases per 10,000 prenatally exposed births.
- Results when using women exposed to other antiemetics as the reference group were consistent with the main analyses (adjusted RR for cardiac malformations, 1.01; 95% CI, 0.92-1.12; for oral clefts, adjusted RR, 1.32; 95% CI, 1.03-1.70; and for any congenital mal- formation RR, 1.00; 95% CI, 0.95-1.05
- The findings generally were not sensitive to changes in the exposure definition (≥2 prescriptions, 6-12 weeks)
Strengths:
- Unlike prior studies where exposure assessment was based on women’s self-reporting, the use of medical records from a large well-known database eliminates the self-reporting bias that may occur when women give birth to an infant with a congenital malformation
- The use of the MAX database and more specifically the prospective collection of information on medications filled eliminates potential recall bias
- Sensitivity analyses were used to test the robustness of the primary results
- The authors quantified the potential for selection bias due to the restriction of the cohort to livebirths using a most extreme scenario
- To further reduce the likelihood of confounding variables, patients taking other anti-emetics were used as alternative reference groups in additional analyses and thus lead to three total groups being compared
- Unexposed women
- Ondansetron-exposed women
- Women exposed to other antiemetics
- Avoided contaminating reference group by excluding women who filled prescription 3 months before start of pregnancy as they could still have the medication available for ingestion after the start of their pregnancy
- Looked at maternal record for congenital abnormalities because Medicaid claims are sometimes recorded under the mother before the infant’s eligibility has been processed
- Considered a very broad range of potential confounders and proxies for confounders (such as treatment indications, demographics, maternal conditions, concomitant medication use, etc.)
- Implemented a confirmatory analysis of 200 empirically defined covariates in addition to the prespecified covariates to account for potential residual confounding.
Limitations:
- Unmeasured covariates can serve to be unknown confounding variables that skew the data. This is a common and well-known limitation of using a propensity score.
- Prevalence at birth was used as a proxy for absolute risk under the assumption that only a small proportion of fetuses with non-syndromic defects would die or be terminated in utero. Therefore, the actual risk may therefore be slightly bigger
- Groups were not well balanced as women in the Ondansetron-exposed group had more markers of comorbid illness and disease severity in the unadjusted analysis
- Filling a prescription does not necessarily mean the patient actually took the medication, especially if it was only filled once.
- This cohort of pregnancies was restricted to live births and thus severe congenital malformations that resulted in the loss or termination of the pregnancy were missed.
- Furthermore, using a Medicaid database composed of primarily disadvantaged women limits the external validity of this study as many countries other than the United States have a form of universal healthcare
- The authors did not account for patients’ prior experiences with Ondansetron and how that may skew the data, this is especially the case if Ondansetron was taken before pregnancy.
- The time period of this study being 2000 to 2013 may also contribute to selection bias.
- There may be some significant bias favoring the use of Ondansetron as several of the authors have received grants from GlaxoSmithKline, the sole creator of Ondansetron from the mid-1980s till 2006.
Discussion:
- This study greatly expands on the currently available evidence which was limited to a cohort of 1349 exposed women and a prior case-control study which showed no risk based on 11 exposed cases.5-7
- It is important to remember that this study investigates the potential long-term effects of an entire Ondansetron prescribed over several days during the first trimester. These effects may not be applicable to a single one-time (or two) dose administered in the emergency department.
- When the authors used women, who were exposed to other antiemetics as the reference group and compared them to the main analysis of Ondansetron, there was no significant difference. Additionally, the results were similar across individual antiemetic agents
- The authors redefined exposure to having filled 2 or more ondansetron prescriptions to evaluate the potential effect of mismatch exposure and to capture patients who filled AND completed taking their first prescription.
- Their redefinition of the exposure window to 6-12 weeks was justified as this being the period of greatest sensitivity to teratogens for oral clefts. The findings were not sensitive to changes in either the exposure or exposure window redefinition.
- Propensity scoring helps limit confounding variables however a significant amount of statistical manipulation, as evident by the multiple analyses and several scoring levels, was performed for the authors to reach their conclusion.
- There is a component of personal experiential bias that has been omitted by the authors. A patient who took Ondansetron before while pregnant with successful relief of her symptoms and no harm to her newborn, may be more inclined to take it again disregarding the presumed congenital malformation risks. The authors made no mention regarding the exclusion of these patients or even if the database had this kind of information.
- The authors justify their use of the 2000 to 2013 time period as being the most recent data available at the time of the study conduct however it’s unclear why they only went as far back as 2000 when Ondansetron has been around since the mid-1980s.
- The use of a primarily disadvantaged cohort (ie. Medicaid database) cannot be overlooked. Had this database come from another country, other than the United States, where a more unified healthcare system exists with easier access to databases and records, the results could likely be very different.
- Lastly an important point that does not seem to be addressed by the authors is the individual patient’s overall clinical picture. Although the risk of oral cleft is small, it is important to always consider both the short-term and long-term benefits and potential harms of any and all interventions as well as how they apply to each unique patient.
Author’s Conclusions:
- “Among newborns of mothers enrolled in Medicaid, first-trimester exposure to ondansetron was not associated with cardiac malformations or congenital malformations overall after accounting for measured confounders but was associated with a small increased risk of oral clefts.”
Our Conclusion:
- Given that it has not been ethically possible to perform an RCT on the effect of Ondansetron on congenital malformations, this study provides us with some additional helpful information on the potential risks of congenital malformations when Ondansetron is used in pregnancy. There are many biases and limitations to this study which may limit its findings, however with this being one of the largest databases currently available, it supports the safety and use of Ondansetron for the cessation of nausea and vomiting in the first trimester of pregnancy.
Clinical Bottom Line:
- Ondansetron use in early pregnancy was not shown in this study to be associated with an increase in cardiac malformations or congenital malformations overall, however there may be a small increased association of oral clefts. IV ondansetron is therefore a safe option, when needed and this study should provide reassurance about ondansetron’s safety in the first trimester of pregnancy.
References:
- Committee on Practice Bulletins-Obstetrics. ACOG Practice Bulletin No. 189: Nausea And Vomiting Of Pregnancy. Obstet Gynecol. 2018 PMID: 29266076
- Huybrechts KF, et al. Association of Maternal First-Trimester Ondansetron Use With Cardiac Malformations and Oral Clefts in Offspring. JAMA. 2018. PMID: 30561479
- Taylor LG, et al. Antiemetic use among pregnant women in the United States: the escalating use of ondansetron. Pharmacoepidemiol Drug Saf. 2017. PMID: 28220993
- Palmsten K, Huybrechts KF, Mogun H, et al. Harnessing the Medicaid Analytic eXtract (MAX) to Evaluate Medications in Pregnancy: Design Considerations. PLoS One. 2013; PMID: 23840692
- Anderka M, et al. Medications used to treat nausea and vomiting of pregnancy and the risk of selected birth defects. Birth Defects Res A Clin Mol Teratol. 2012. PMID: 22102545
- Parker SE, et al.; National Birth Defects Prevention Study. Ondansetron for Treatment of Nausea and Vomiting of Pregnancy and the Risk of Specific Birth Defects. Obstet Gynecol. 2018. PMID: 29995744
- Danielsson B, et al. Use of ondansetron during pregnancy and congenital malformations in the infant. Reprod Toxicol. 2017. PMID: 25450422
For More on This Topic Checkout:
- PharmERToxGuy: IV Ondansetron in the First Trimester of Pregnancy
Post Peer Reviewed By: Salim R. Rezaie, MD (Twitter: @srrezaie)
The post Association of Maternal First-Trimester Ondansetron Use with Cardiac Malformations and Oral Clefts in Newborns appeared first on REBEL EM - Emergency Medicine Blog.
