
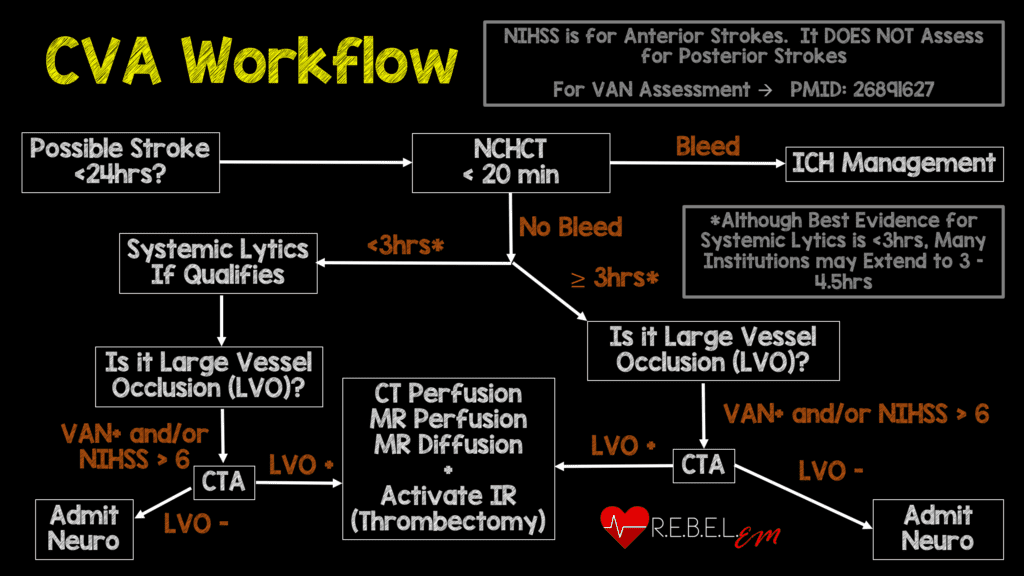
 Background: The publication of the MR CLEAN trial in January 2015 changed the face of ischemic stroke care. This was the first study demonstrating a benefit to endovascular treatment of a specific subset of ischemic stroke patients: those with a large vessel occlusion (LVO) presenting within 6 hours of symptom onset. MR CLEAN was followed by a flurry of publications seeking to replicate and refine treatment as well as expand the window for treatment. The REBEL EM team reviewed this literature back in 2018 and, with the help of Dr. Evie Marcolini, created a workflow (see CVA Workflow below).
Background: The publication of the MR CLEAN trial in January 2015 changed the face of ischemic stroke care. This was the first study demonstrating a benefit to endovascular treatment of a specific subset of ischemic stroke patients: those with a large vessel occlusion (LVO) presenting within 6 hours of symptom onset. MR CLEAN was followed by a flurry of publications seeking to replicate and refine treatment as well as expand the window for treatment. The REBEL EM team reviewed this literature back in 2018 and, with the help of Dr. Evie Marcolini, created a workflow (see CVA Workflow below).
One major component of LVO management is the use of systemic thrombolytics in patients presenting within the current thrombolytic treatment window prior to endovascular intervention. However, it’s unclear if systemic thrombolytic administration results in better outcomes or if it simply exposes the patient to increased risks at a higher cost. Limited evidence questions the utility of the current approach with lytics + endovascular therapy (Phan 2017, Rai 2018). In 2020, we reviewed an article by Yang and colleagues that demonstrated non-inferiority to an endovascular intervention only approach (with a 20% non-inferiority lower limit) (REBEL EM). Recently, two more studies have been published on this topic.
Clinical Question: Is endovascular mechanical thrombectomy alone non-inferior to endovascular therapy + systemic thrombolytics in the treatment of patients with large vessel occlusion (LVO) strokes presenting within 4.5 hours of onset?
The DEVT Trial
Article#1: Wenjie Z et al. Effect of endovascular treatment alone vs intravenous alteplase plus endovascular treatment on functional independence in patients with acute ischemic stroke: the DEVT randomized clinical trial. JAMA 2021; 325(3): 234-43. PMID: 33464335
Population: Patients > 18 years of age presenting within 4.5 hours of ischemic stroke symptom onset, eligible for IV alteplase treatment and with cerebral vascular occlusion on CT angiography (CTA) or magnetic resonance angiography (MRA) of the intracranial internal carotid artery or middle cerebral artery (first segment). Patients had to have small penumbra as well.
Outcomes:
- Primary: Proportion of patients achieving functional independence – modified Rankin scale (mRS) 0-2 assessed at 90 days after randomization looking for non-inferiority (margin -10%)
-
Secondary:
- Distribution of 90-day mRS scores
- Rates of excellent (mRS 0-1) outcomes
- Successful reperfusion on postprocedural angiography
- Vessel reperfusion on CTA or MRA within 48 hours of treatment
- Change in NIHSS score
- EQ-5D-5L at 90 days
-
Safety Outcomes
- Incidence of symptomatic intracranial hemorrhage within 48 hours
- 90-day mortality
- Procedure-associated complications
Intervention: Endovascular treatment alone
Control: Endovascular thrombectomy + systemic alteplase 0.9 mg/kg
Design: Multicenter, randomized, non-inferiority, non-blinded prospective study at 33 stroke centers in China
Excluded:
- Prior disability before the stroke (mRS > 2)
- Contraindication to intravenous alteplase including intracranial hemorrhage
Primary Results:
-
- 509 patients assessed across 33 centers over 24 months
- 235 patients enrolled
- 117 to thrombectomy-alone + 118 to combination treatment
- 3 patients in each group did not receive treatment they were randomized to
- Median age = 70 years
- Median NIHSS score = 16
- Average time of onset to randomization was 169 minutes
- Average time of onset to puncture was 205 minutes
Critical Findings:
-
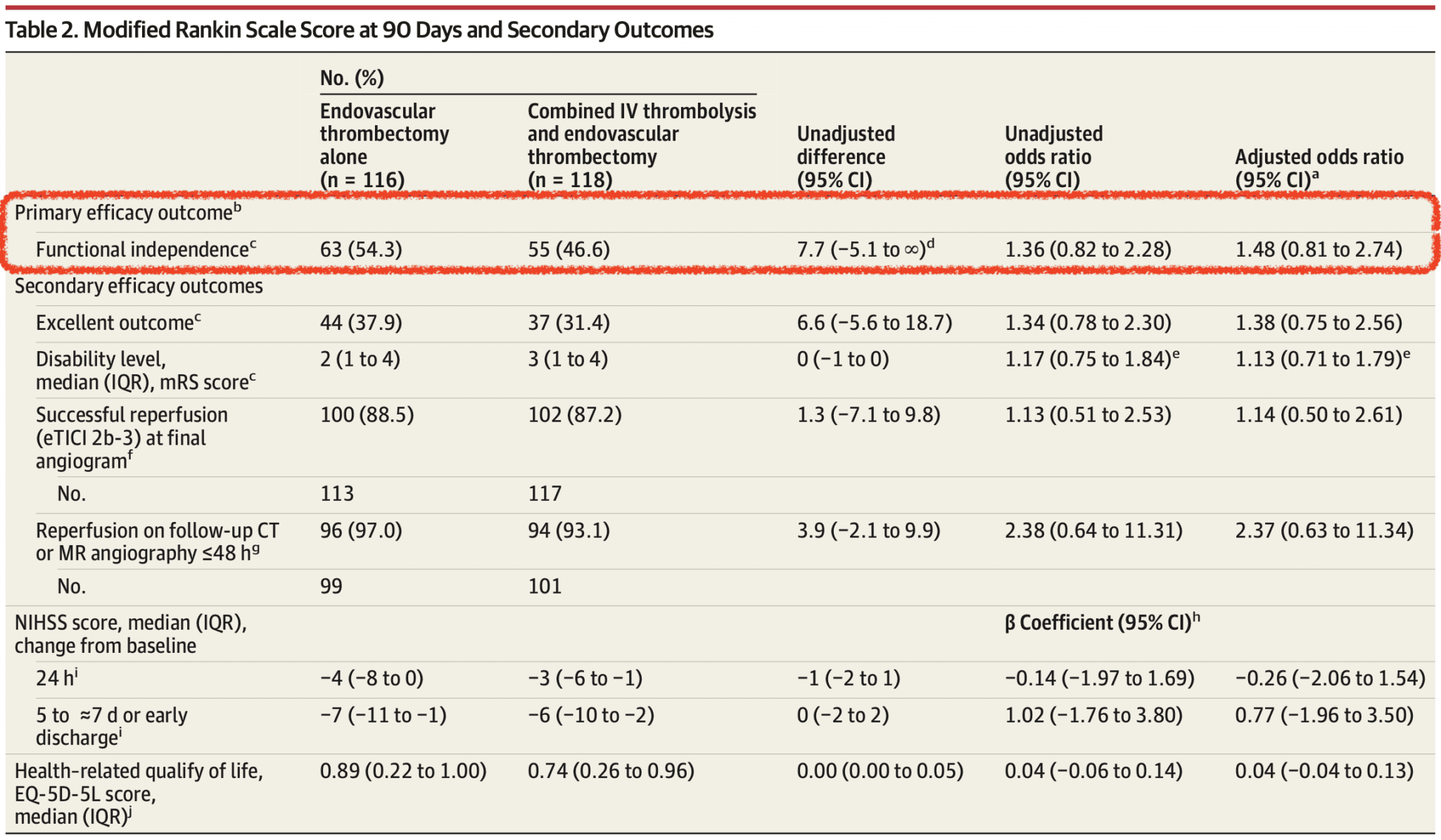
Primary Outcome (mRS 0-2 at 90 days)
- Endovascular Alone vs Combo: 54.3% vs 46.6%
- Absolute difference 7.7%
- 97.5% 1-sided CI: -5.1 to Infinity
- Demonstrates non-inferiority for endovascular alone
- No significant difference in secondary outcomes of symptomatic intracerebral hemorrhage and 90-day mortality
- No difference in symptomatic intracerebral hemorrhage between groups (6.1% vs 6.8%)
- No difference in patients who died within 90d (17.2% vs 17.8%; -0.05% Difference; 95% CI -10.3% to 9.2%)
- Clot migration occurred less frequently in EVT alone group (17.7% vs 23.9%)
- No significant differences in complications or serious adverse events but there was a trend toward decreased rate of procedure associated complications in the EVT alone group (30.1% vs 41.0%)
Strengths:
- Study asks an important clinical question that has implications for patient outcomes as well as resource utilization
- The study was performed across multiple institutions which increases external validity
- Primary outcome is patient centered (function) as opposed to disease oriented (ie reperfusion)
- Randomization was adequately performed, and baseline characteristics are well balanced
- All sites were experienced with endovascular therapy – required to perform at least 50 procedures in last year
- Although patients and clinicians were not blinded to the use of alteplase, both outcome assessors and radiologists reviewing scans for reperfusion and infarct size were blinded to treatment arm
- Outcome assessment at 90 days was performed by two blinded neurologists based on video or audio recordings from outpatient clinic
- No patients lost to 90 day follow up
Limitations:
- The study was stopped early due to pre-planned interim analysis that demonstrated non-inferiority. Only 1/4 of the planned enrollment was completed
- Though the mRs is a well-accepted outcome measure, there is some subjectivity to it which threatens the validity of the data
- The study was only performed in China which decreases external validity
- Patients were not enrolled consecutively
- Secondary outcome of symptomatic intracerebral hemorrhage is a subjective measure which could be influenced by the absence of blinding
- The number of intracranial internal carotid artery occlusions were not as common (i.e. 15%) which could be one of the reasons functional outcomes were so high in this trial
Authors Conclusions: “Among patients with ischemic stroke due to proximal anterior circulation occlusion within 4.5 hours from onset, endovascular treatment alone, compared with intravenous alteplase plus endovascular treatment, met the prespecified statistical threshold for noninferiority for the outcome of 90-day functional independence. These findings should be interpreted in the context of the clinical acceptability of the selected noninferiority threshold.”
Our Conclusions: Endovascular therapy alone met the non-inferiority threshold when compared to combination therapy. However, this was a small trial that was stopped early and should be taken in context with all the available literature on the subject.
The SKIP Trial
Article #2: Suzuki K et al. Effect of mechanical thrombectomy without vs with intravenous thrombolysis on functional outcome among patients with acute ischemic stroke: The SKIP randomized clinical trial. JAMA 2021; 325(3): 244-53. PMID: 33464334
Population: Patients > 18 years and ≤85 years of age presenting within 4.5 hours of ischemic stroke symptom onset, eligible for IV alteplase treatment and with cerebral vascular occlusion on CT angiography (CTA) or magnetic resonance angiography (MRA) of the intracranial internal carotid artery or middle cerebral artery (first segment). Patients had to have small penumbra as well (based on ASPECTS score).
Outcomes:
- Primary: Proportion of patients achieving function independence – modified Rankin scale (mRS) 0-2 assessed at 90 days after randomization looking for non-inferiority (non-inferiority margin odds ratio 0.74)
-
Secondary:
- Shift analysis of disability levels on the mRS
- mRS score of 5-6
- mRS 0-1
- mRS 0-3
- Mortality at 90 days
- Successful reperfusion
-
Safety Outcomes
- Any intracerebral hemorrhage at 36 hours
- Symptomatic intracerebral hemorrhage
- 90-day mortality
- Procedure-associated complications
Intervention: Endovascular treatment alone
Control: Endovascular thrombectomy + systemic alteplase 0.6 mg/kg
Design: Multicenter, randomized, non-blinded non-inferiority, prospective study in Japan
Excluded:
- Contraindication for contrast agent or endovascular therapy
- Contraindication for IVT
- Presence of severe renal disorder (patients undergoing dialysis can be included)
- Pregnancy or possibility of pregnancy
- Unlikely to complete the study
- Judged incompatible with the study by the investigators
Primary Results:
-
- 204 patients randomized
- 101 to thrombectomy-alone + 103 to combination treatment
- 10 patients excluded after randomization (8 for ASPECTS score too low, 2 for Pre-mRS > 2)
- 194 patients analyzed
- Median age = 74 years
- Median NIHSS score = 19 (thrombectomy alone) vs 17 (combo therapy)
- 204 patients randomized
Critical Findings:
-
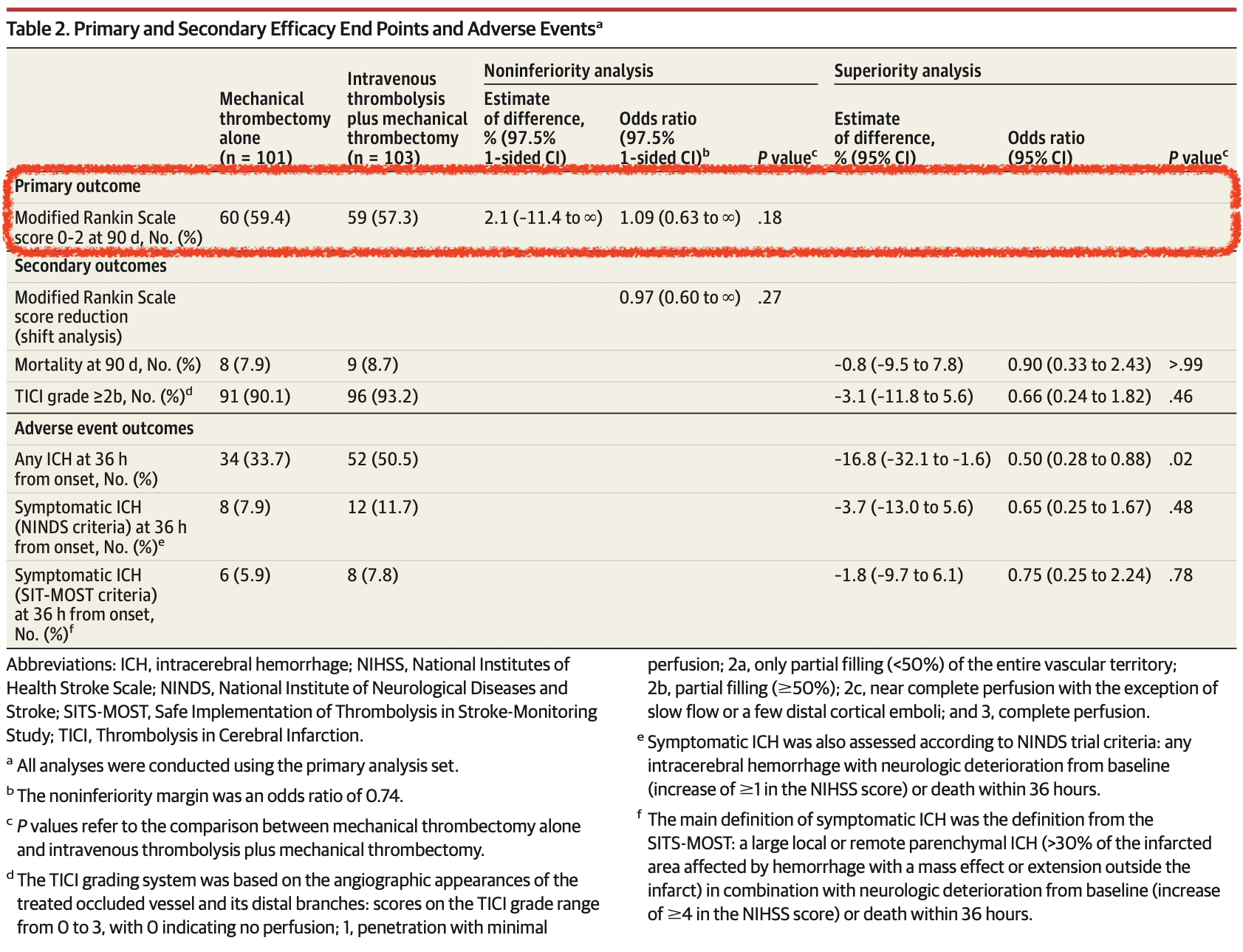
Primary Outcome (mRS 0-2 at 90 days)
- Endovascular Alone vs Combo: 59.4% vs 57.3%
- Absolute difference 2.1%
- 1-sided 97.5% CI: -11.4 to infinity
- Odds ratio: 10.9 (1 sided 97.5% CI 0.63 to infinity)
- Failed to demonstrate non-inferiority of endovascular only approach
- No significant difference in secondary outcomes of symptomatic intracerebral hemorrhage and 90-day mortality
Strengths:
- Study asks an important clinical question that has implications for patient outcomes as well as resource utilization
- The study was performed across multiple institutions which increases external validity
- Primary outcome is patient centered (function) as opposed to disease oriented (ie reperfusion)
- Randomization was adequately performed, and baseline characteristics are well balanced
- Although patients and clinicians were not blinded to the use of alteplase, both outcome assessors and radiologists reviewing scans for reperfusion and infarct size were blinded to treatment arm
- Outcome assessment at 90 days was performed by two blinded neurologists based on video or audio recordings from outpatient clinic
- Secondary and safety outcomes were prespecified which shows these outcomes were determined prior to initiation of investigation
Limitations:
- This is a small trial that appears to be underpowered
- There was a minor difference in the median NIHSS scores (19 vs 17) favoring the combination group
- Though the mRs is a well-accepted outcome measure, there is some subjectivity to it which threatens the validity of the data
- The study was only performed in Japan which decreases external validity
- Patients were not enrolled consecutively
- Outcome assessment performed by physical examination or telephone at 90 days. Assessors were blinded but, as patients were not, there is potential for unintentional unblinding
- Secondary outcome of symptomatic intracerebral hemorrhage is a subjective measure which could be influenced by the absence of blinding
- Used a lower dose of alteplase although prior studies have found that a dose of 0.6 mg/kg is comparable to 0.9 mg/kg (REBEL EM)
- 22 patients (21.4%) in the combination group had EVT prior to thrombolysis which is not what we are comparing. We are comparing thrombolysis 1st then EVT.
Authors Conclusions: “Among patients with acute large vessel occlusion stroke, mechanical thrombectomy alone, compared with combined intravenous thrombolysis plus mechanical thrombectomy, failed to demonstrate noninferiority regarding favorable functional outcome. However, the wide confidence intervals around the effect estimate also did not allow a conclusion of inferiority.”
Our Conclusions: The authors did not demonstrate non-inferiority for thrombectomy alone in comparison to combination treatment.
Discussion:
- We now have three randomized controlled trials (DIRECT-MT, DEVT, SKIP) as well as retrospective, non-randomized data (Phan 2017, Rai 2018) looking at this question.
- Overall, data has been reported on about 1000 patients with the two larger trials demonstrating non-inferiority
- There are additional ongoing trials that will add to the available evidence
- A non-inferiority approach makes sense in answering this question. Finding non-inferiority would mean being able to avoid administration of systemic lytics in this group.
- In the absence of a clear benefit to systemic thrombolytics, their use must be questioned as they increase risk to the patient (intracranial and extracranial hemorrhage, early death) and increase costs (US cost ~ 6.5K)
- The prespecified non-inferiority margins of these three studies were all different. An important aspect of moving towards thrombectomy alone will be considering the acceptable margin
- In all three RCTs, the endovascular arm performed numerically better than the combination arm. This would appear to support a move towards endovascular therapy alone
- All Facilities are NOT Comprehensive Stroke Centers:
- Application of this data to locations where endovasular therapy is only available after transfer to a larger center must be made cautiously. In a comprehensive stroke center, time from door to groin puncture will be shorter than what would be seen if transfer required. Whether this makes a difference regarding use of systemic lytics is unknown.
- Both SKIP and DEVT were at stroke centers not community centers. Many hospitals will not have access to advanced imaging and may not be able to identify which patients are eligible for EVT without transfer. These centers may need to give alteplase prior to transfer as there is usually delay in getting mechanical thrombectomy.
- Both studies excluded patients with MCA M2 segment occlusions (were included in other endovascular studies) and therefore results may not generalize to this population
- DEVT had intracranial occlusion 15% and SKIP had it 37.7%. This may be one of the reasons we see differences in outcomes.
Potential to Impact Current Practice: The majority of available, high-quality data supports endovascular therapy alone for patients with large vessel occlusion and a significant penumbra with a small infarct core. While we await the results of additional studies, it’s time to reassess the use of systemic thrombolytics in this group of patients when endovascular care is readily available.
For More on This Topic Checkout:
- REBEL EM: Endovascular Therapy for Acute Ischemic Stroke
- REBEL EM: The DIRECT-MT Trial: Are Systemic Lytics Necessary in LVO Treatment?
References:
- Phan K et al. Endovascular thrombectomy alone versus combined with intravenous thrombolysis. World Neurosurg 2017; 108: 850-8. PMID: 28823660
- Rai AT et al. Intravenous thrombolysis before endovascular therapy for large vessel strokes can lead to significantly higher hospital costs with our improving outcomes. J Neurointerv Sure 2018; 10(1): 17-21. PMID: 28062805
- Yang P et al. Endocascular thrombectomy with or without intravenous alteplase in acute stroke. NJEM 2020. PMID: 32374959
- Wenjie Z et al. Effect of end-vascular treatment alone vs intravenous alteplase plus end-vascular treatment on functional independence in patients with acute ischemic stroke: the DEVT randomized clinical trial. JAMA 2021; 325(3): 234-43. PMID: 33464335
- Suzuki K et al. Effect of mechanical thrombectomy without vs with intravenous thrombolysis on functional outcome among patients with acute ischemic stroke: The SKIP randomized clinical trial. JAMA 2021; 325(3): 244-53. PMID: 33464334
Post Peer Reviewed By: Salim R. Rezaie, MD (Twitter: @srrezaie)
The post The DEVT + SKIP Trials: Does Systemic Thrombolysis Prior to Endovascular Treatment Improve Outcomes in Large Vessel Occlusion Strokes? appeared first on REBEL EM - Emergency Medicine Blog.
