
Background Information:
 Delirium is a common and serious condition in patients in the intensive care unit (ICU). It is estimated to affect 30-50% of patients in the ICU and haloperidol is the most frequently used agent in treatment (3). One of the most common methods of assessing delirium in the ICU is using the Confusion Assessment Method for Intensive Care Unit (CAM-ICU) which has been found to have a sensitivity of 80% and a specificity of 95.9% (6). A previous similar study, the Modifying the Impact of ICU-Associated Neurological Dysfunction (MIND-USA) trial seeked to study whether antipsychotics (haloperidol or ziprasidone) are better or worse than placebo in delirious ICU patients. The authors of the AID-ICU study aim to determine whether haloperidol leads to a significantly greater number of days alive and out of the hospital at 90 days than placebo in ICU patients with delirium.
Delirium is a common and serious condition in patients in the intensive care unit (ICU). It is estimated to affect 30-50% of patients in the ICU and haloperidol is the most frequently used agent in treatment (3). One of the most common methods of assessing delirium in the ICU is using the Confusion Assessment Method for Intensive Care Unit (CAM-ICU) which has been found to have a sensitivity of 80% and a specificity of 95.9% (6). A previous similar study, the Modifying the Impact of ICU-Associated Neurological Dysfunction (MIND-USA) trial seeked to study whether antipsychotics (haloperidol or ziprasidone) are better or worse than placebo in delirious ICU patients. The authors of the AID-ICU study aim to determine whether haloperidol leads to a significantly greater number of days alive and out of the hospital at 90 days than placebo in ICU patients with delirium.
Paper: Andersen-Ranberg NC,et al; AID-ICU Trial Group. Haloperidol for the Treatment of Delirium in ICU Patients. N Engl J Med. 2022 Dec 29. PMID: 36286254
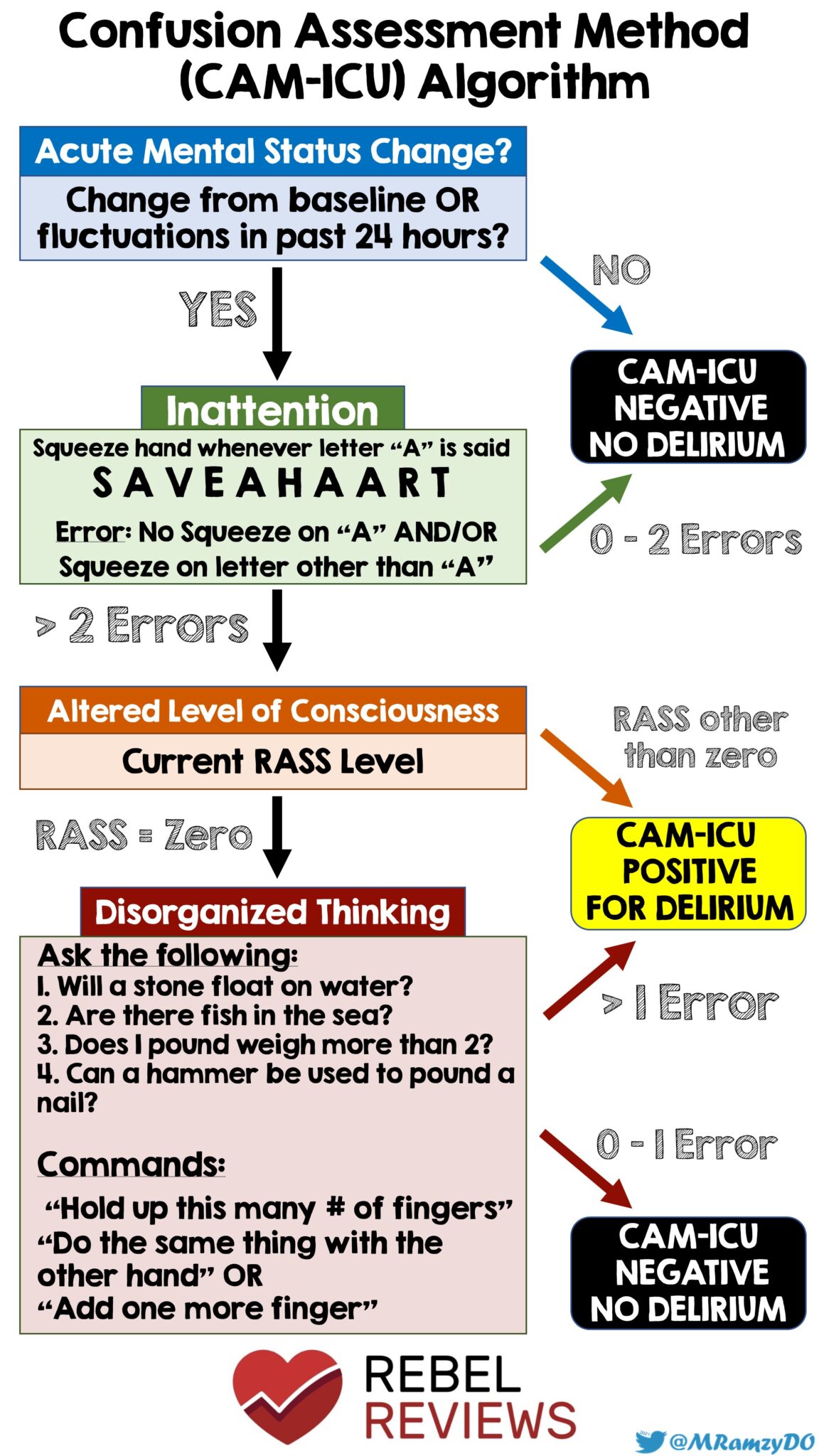
Figure 1: CAM Algorithm for Delirium (Source: @MRamzyDO)
Clinical Question:
- Does the use of haloperidol lead to a significantly greater number of days alive and out of the hospital at 90 days than placebo in ICU patients with delirium?
What They Did:
- Large, blinded placebo controlled trial across 18 general ICU’s in Denmark, Finland, the United Kingdom, Italy and Spain between June 14,2018 and April 9,2022.
- Patients 18 years or older being treated for an acute condition in the ICU were screened for delirium at least twice daily by clinical staff using either the Confusion Assessment Method for the ICU (CAM-ICU) or the Intensive Care Delirium Screening Checklist (ICDSC)
- Positive for Delirium if:
- Positive CAM-ICU assessment
- At least 4 symptoms for delirium on ICDSC assessment
-
Patients were randomized to one of the following two groups:
- Control group: patients received the placebo in the form of isotonic saline
- Experimental group: patients received IV haloperidol 2.5mg three times daily plus 2.5mg as needed to a total maximum daily dose of 20mg.
- Trial design allowed for as needed administration of haloperidol or placebo and the use of rescue medications such as propofol, alpha 2 agonist and benzodiazepines so that clinical staff could safely care for patients with delirium.
Inclusion Criteria:
- Patients 18 years of age or older admitted to an ICU for an acute condition AND
-
Received a positive result on a screening test for delirium using either:
- Confusion Assessment Method for the ICU (CAM-ICU) or
-
Intensive Care Delirium Screening Checklist (ICDSC):
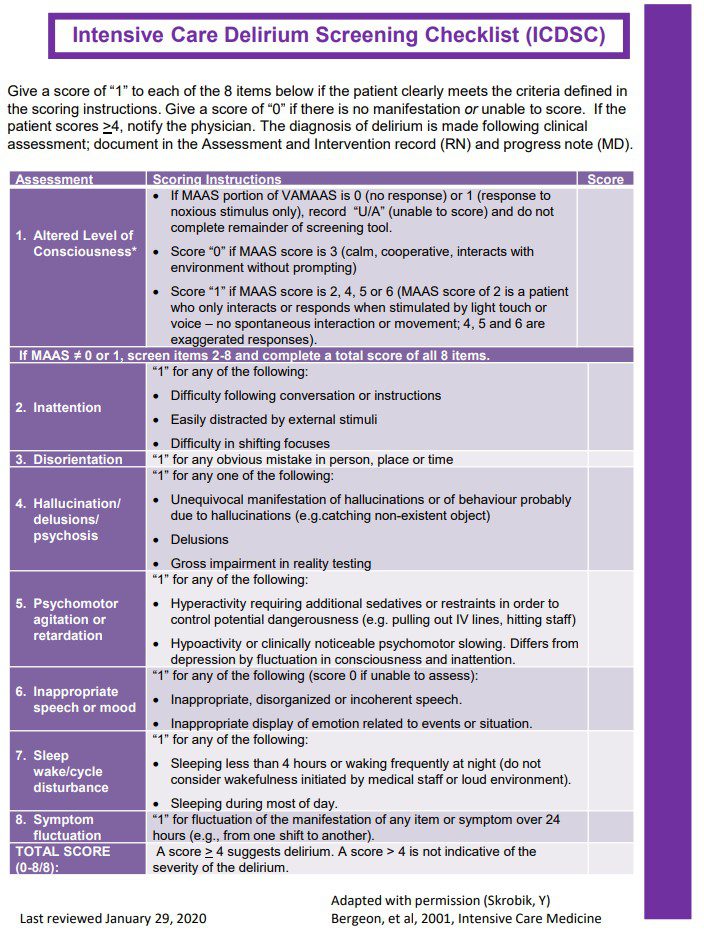
Figure 2: ICDSC Checklist (Source: Bergeon, et al, 2001, Intensive Care Medicine)
Exclusion Criteria:
- Did not have consent provided (either by patient or surrogate)
- Received an antipsychotic agent in the ICU
- Contraindication to Haloperidol
- Language barrier, deafness or blindness precluded adequate assessment of delirium
- Presence of delirium tremens
- Patients who were involuntarily admitted to the hospital
Outcomes:
Primary
- The primary outcome of the study was the number of days alive and out of the hospital within 90 days after randomization
Secondary
- Number of days alive without delirium of coma in the ICU at 90 days
- number of days alive without mechanical ventilation at 90 days
- number of patients with one or more serious adverse reaction to haloperidol in the ICU
- Total number of serious adverse reactions to haloperidol in the ICU
- Number of patients receiving rescue medication
- Number of days with rescue medication per patient
Results:
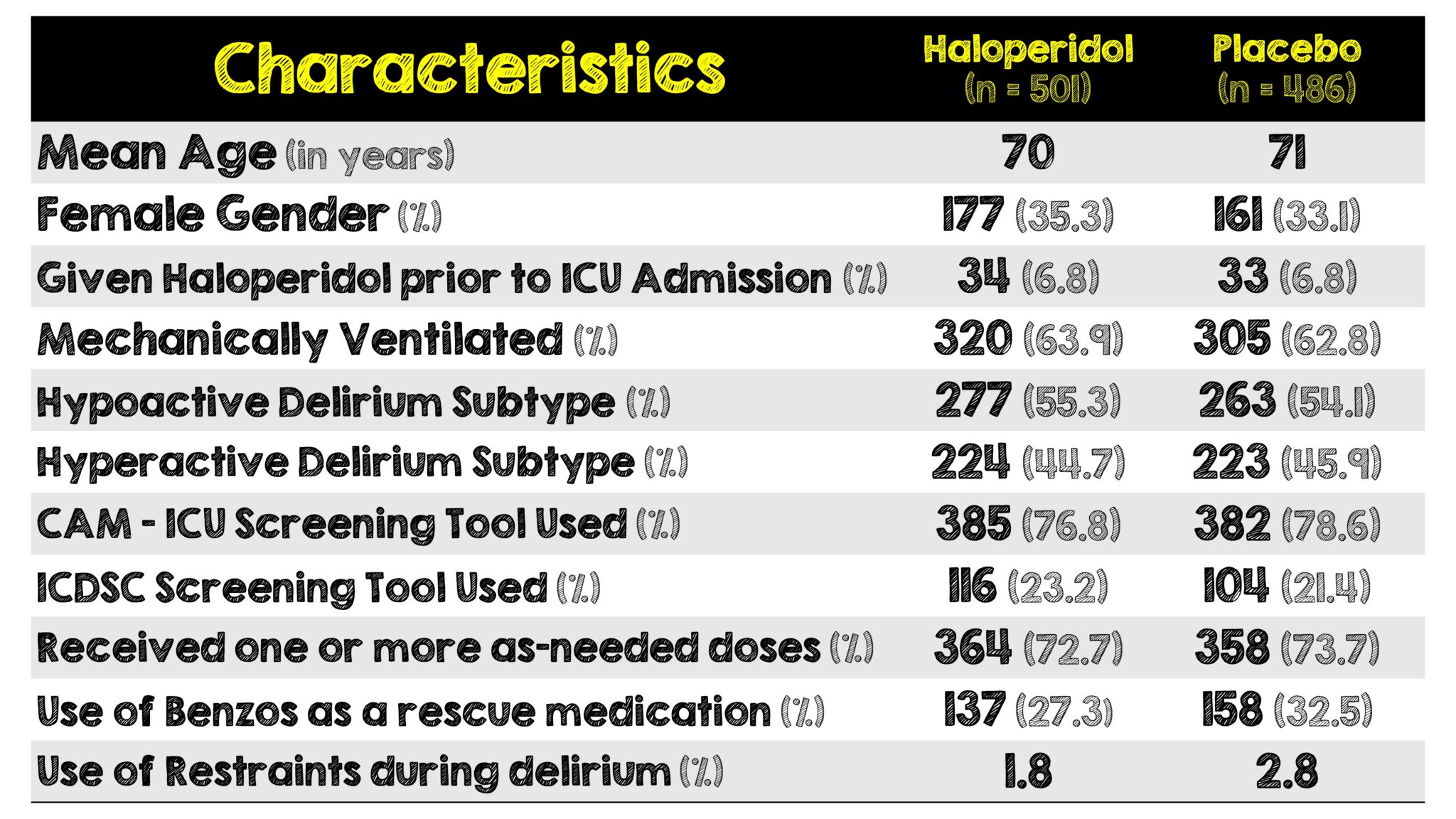
- 166 (33.1%) of patients in the Haloperidol group and 143 (29.4%) in the placebo received benzodiazepines in the hospital before randomization. No mention was made of what kind, how much or how often
Critical Results:
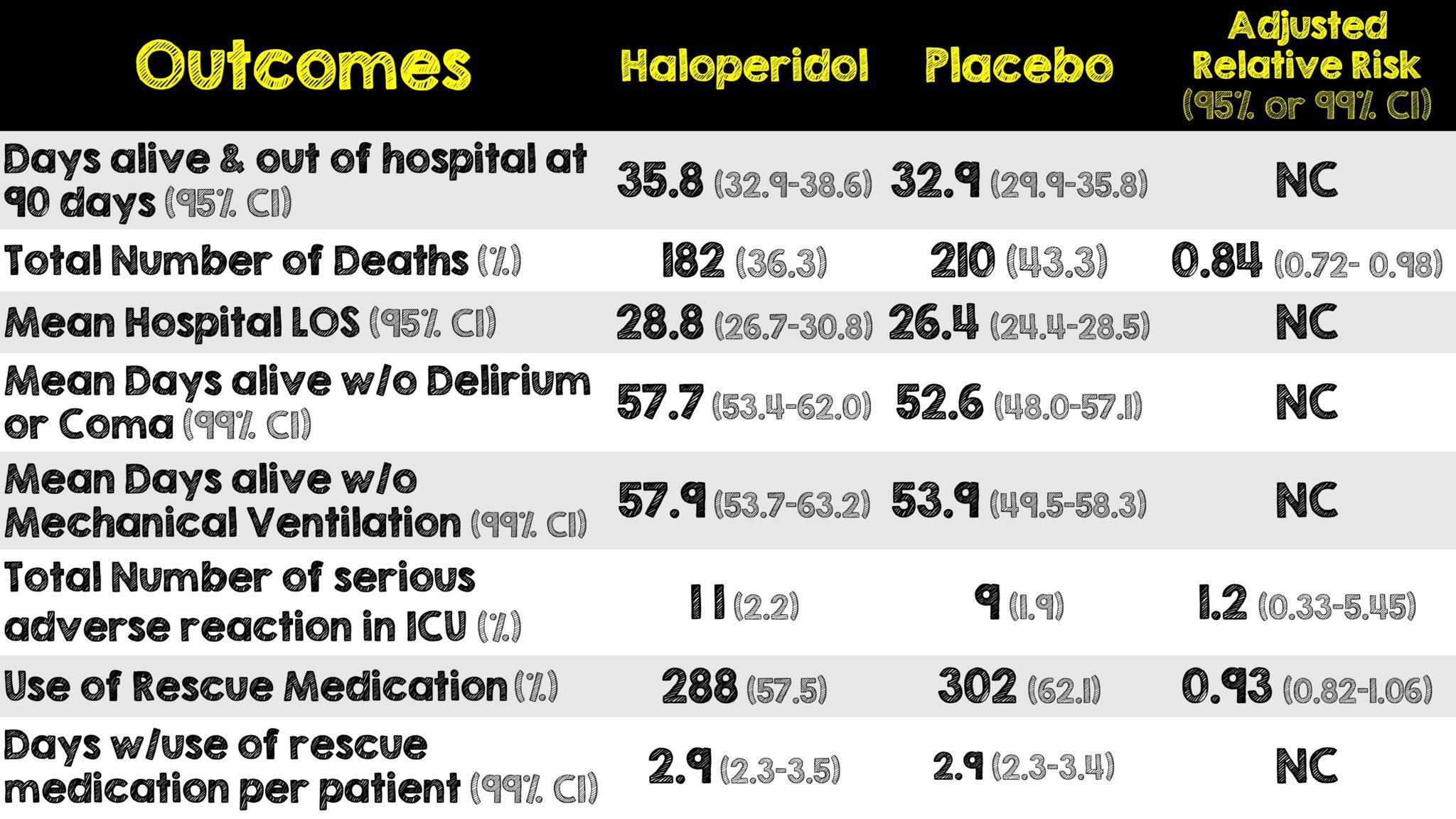
- Median time from ICU admission to randomization was 3.9 days (1.8 – 9.7 IQR) in the haloperidol group and 4.1 days (1.6 – 8.9 IQR) in the placebo group.
Strengths:
- Blinded, placebo controlled design with a large sample size and high level of completeness of data
- Well balanced groups
- Very little loss to follow-up for primary outcome
- Funding organizations had no influence on study design, conduct including collection, analysis or reporting of the data
- Clinicians, patients, investigators, assessors, statisticians and members of the data and safety monitoring committee were unaware of trial group assignments
- Haloperidol and placebo were in identical ampules with identical labeling. Both solutions were colorless and indistinguishable from each other
- Utilized validated diagnostic tools for assessment of delirium
- Patient oriented outcome
- Asks and attempts to answer a clinically relevant question
- Differentiated between hypoactive and hyperactive delirium
- PRN and rescue meds to ensure staff safety
Limitations:
- Limited generalizability of findings due to limited number of patients from international sites
- No detailed information collected on sedatives, pain medications or non-pharmacologic interventions administered to patients which could confound results
- Lower enrollment of patients with hypoactive delirium compared to hyperactive delirium
- High rate of total protocol violations
- Large number of patients were excluded at screening due to prior antipsychotic use and thus could introduce a selection bias
- The method of differentiating between hypoactive and hyperactive delirium was not explicitly explained in the article, however patients were categorized into each subtype during randomization
Discussion:
- Delirium is estimated to affect 30-50% of patients being treated in the ICU (3). Approximately half of the ICU patients with delirium received haloperidol in a cohort study published in 2018 (5) however the use of haloperidol is not supported by practice guidelines due to evidence of its effect being limited (6).
- Results of this trial were very similar to the MIND-USA trial where both investigations observed no significant difference in the number of days alive without delirium or coma, or in 90-day mortality rates between the control and haloperidol groups, with minimal adverse effects reported in the latter.
- The findings from the AID-ICU trial supports those found in the MIND-USA trial
-
The AID-ICU trial in comparison to MIND-USA trial had:
- Larger sample size
- More inclusive criteria
- Older patient demographic
- Reduced use of open label antipsychotic medications
- Higher proportion of patients with hyperactive delirium
- Lots of patients got benzodiazepines prior to randomization (33.1% in Haldol and 29.4% in the placebo group). We unfortunately have no idea how much, what kind or how often. Benzos in elderly patients can worsen delirium so this certainly may have played a role in the overall results
- There authors sought a 15% reduction of death from the single intervention of Haloperidol which is pretty high and may be one of the reasons statistical significance was not achieved in this trial
- The lack of breaking patients into phenotypes makes it hard to know what to do with the results of this trial. Over half the patients (540) were hypoactive delirium and we typically don’t see a benefit in treating these patients with antipsychotics
- The median daily dose of haloperidol given was 8.3mg over 3.6 days in the haloperidol group and the median daily dose of haloperidol given in the placebo group was 9.0mg over 3.3 days which isn’t that big of a difference and because there was no real separation in the median daily dose between intervention and control it would be unrealistic to find a difference in the primary outcome
- With the above mentioned, it may be time to start investigating specific therapies for each type of delirium and their benefit (if any) over the current standard of care. Hypoactive delirium is harder to recognize and harder to treat so more investigative efforts should be put towards this specific type
Author’s Conclusions:
- Among patients in the ICU with delirium, treatment with haloperidol did not lead to a significantly greater number of days alive and out of the hospital at 90 days than placebo.
Our Conclusion:
- With the addition of the AID-ICU trial to the MINDS-USA trial we now have additional data investigating the use of haloperidol for ICU delirium. The use of haloperidol in managing delirium in ICU patients did not result in a reduction of mortality rates or length of hospital stay. It’s time to start differentiating the types of delirium and we need to investigate the specific therapies (if any) that could help one or the other.
Clinical Bottom Line:
Although this study did not result in a reduction of mortality rates or length of hospital stay with minimal adverse effects, it’s difficult to draw this kind of a firm conclusion given the heterogenous nature of delirium with it’s different subtypes. This study grouped all the patients into one and then attempted to look for a difference which is not entirely helpful. Some patients will be helped, others harmed, but this kind of grouping does not allow us to determine a difference. We’ll continue to use haloperidol for hyperactive delirious patients in the ICU until additional evidence further differentiating this complex disease process indicates otherwise.
Guest Post By:

Gopu Premrajan, MD
PGY-2, Emergency Medicine Resident
RWJBH Community Medical Center, Toms River, NJ
Gopu.premrajan@rwjbh.org

Mark Ramzy, DO
Emergency Medicine Attending and Cardiothoracic Intensivist
Clinical Assistant Professor of Emergency Medicine
RWJBH Community Medical Center, Toms River, NJ
Twitter: @MRamzyDO
REFERENCES:
- Andersen-Ranberg NC,et al; AID-ICU Trial Group. Haloperidol for the Treatment of Delirium in ICU Patients. N Engl J Med. 2022 Dec 29. PMID: 36286254
- Girard TD et al. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018; PMID: 30346242
- Salluh JI et al. Outcome of delirium in critically ill patients: systematic review and meta-analysis. BMJ 2015; PMID: 26041151
- Collet MO et al. Prevalence and risk factors related to haloperidol use for delirium in adult intensive care patients: the multinational AID-ICU inception cohort study. Intensive Care Med 2018; PMID: 29767323
- Devlin JW et al. Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU. Crit Care Med. 2018; PMID: 30113379
- Gusmao-Flores et al. The confusion assessment method for the intensive care unit (CAM-ICU) and intensive care delirium screening checklist (ICDSC) for the diagnosis of delirium: a systematic review and meta-analysis of clinical studies. Crit Care 16, R115 (2012). PMID: 22759376
For More Thoughts on This Topic Checkout:
-
- REBEL EM: MINDS-USA Trial
- EMCrit: Antipsychotics and Delirium
Post Peer Reviewed By: Salim Rezaie, MD (Twitter: @Srrezaie)
The post AID-ICU Trial: Haloperidol for Treatment of ICU Delirium appeared first on REBEL EM - Emergency Medicine Blog.
