
 Background: Diphenhydramine, a first-generation antihistamine, is the most common pharmacologic agent used to treat acute allergic reactions. Despite being highly effective in treating acute allergic reactions, first-generation H1 antihistamines cross the blood-brain barrier and bind to H1 receptors, which can lead to undesirable side effects, including drowsiness, sedation, fatigue, and decreased cognition (Church MK 2010). In addition, H1 antihistamines non-selectively bind to other receptors (muscarinic, serotonin, and alpha-adrenergic receptors), which can lead to additional side effects such as urinary retention, constipation, and dry mouth.
Background: Diphenhydramine, a first-generation antihistamine, is the most common pharmacologic agent used to treat acute allergic reactions. Despite being highly effective in treating acute allergic reactions, first-generation H1 antihistamines cross the blood-brain barrier and bind to H1 receptors, which can lead to undesirable side effects, including drowsiness, sedation, fatigue, and decreased cognition (Church MK 2010). In addition, H1 antihistamines non-selectively bind to other receptors (muscarinic, serotonin, and alpha-adrenergic receptors), which can lead to additional side effects such as urinary retention, constipation, and dry mouth.
Second-generation H1 antihistamines (cetirizine, loratadine, and fexofenadine) do not cross the blood-brain barrier as easily (Bernstein J 2018). They are less sedating compared to first-generation antihistamines and have fewer associated adverse effects. Moreover, the FDA approved intravenous (IV) cetirizine to treat acute urticaria in 2019.
Clinical Question: Is IV cetirizine noninferior to IV diphenhydramine for the treatment of acute urticaria?
Article: Abella BS et al. Intravenous Cetirizine Versus Intravenous Diphenhydramine for the Treatment of Acute Urticaria: A Phase III Randomized Controlled Noninferiority Trial. Ann Emerg Med 2020. PMID: 32653333
What They Did
- Multi-center, double-blind, randomized, phase 3 clinical trial with a parallel-group, active-controlled, non-inferiority design.
- Patients were randomized in a 1:1 ratio.
- Enrolled in emergency departments and urgent care centers
Population
Inclusion:
-
Patients > 18 years of age who required an antihistamine to relieve acute urticaria with a patient-rated pruritus severity score ≥ 1
- Pruritus severity score of 0-3 (0=none, 1=mild, 2=moderate, 3=severe)
- Acute urticaria caused by a reaction to a current medication (e.g., antibiotics, NSAIDs)
- Acute urticaria with angioedema or anaphylaxis provided that urticaria was still present after initial treatment and alleviation of anaphylaxis symptoms.
Exclusion:
- Presented with acute anaphylaxis, and their acute anaphylactic symptoms had not yet been treated.
- A contraindication, known allergy, or suspected intolerability to the study medication.
- Receipt of an H1 or H2 antagonist or doxepin in the previous 2 hours
- Received corticosteroids in the previous 4 hours
- Epinephrine received in the previous 20 minutes
- Concomitant use of p-glycoproteins inhibitors (including amiodarone, clarithromycin, erythromycin, ketoconazole, quinidine, and saquinavir)
Intervention
10mg of IV cetirizine
Comparator
50mg of IV diphenhydramine
Outcomes
Primary Endpoint:
- Change in patient-rated pruritus score from baseline to 2 hours after treatment administration
Key Secondary Endpoints:
- Percentage of patients who return to any ED or clinic
- The time spent at the treatment center (time from treatment administration to discharge readiness)
Other Secondary Endpoints:
- Physician rated the extent of urticaria/erythema score reduction from baseline at 2 hrs
- Percentage of patients needing rescue medication, pruritus treatment success, effectively treated, returning to normal activity, and symptom recurrence after discharge
- Patient-rated sedation scores
- Adverse events
Results
- 19 study centers participated in the enrollment of patients: 17 emergency departments and 2 urgent care centers
-
268 eligible patients were recruited
- 6 patients were excluded due to lack of IV access (n=3)
- Symptoms resolving before treatment (n=1)
- Screen failure (n=1)
- Study discontinuation before study drug exposure (n=1)
-
262 eligible patients were randomized
- 135 patients were allocated to the IV Diphenhydramine 50mg group
- 127 patients were allocated to the IV Cetirizine 10mg group
- 87% of included patients had urticaria only, and this was balanced between groups
Critical Findings
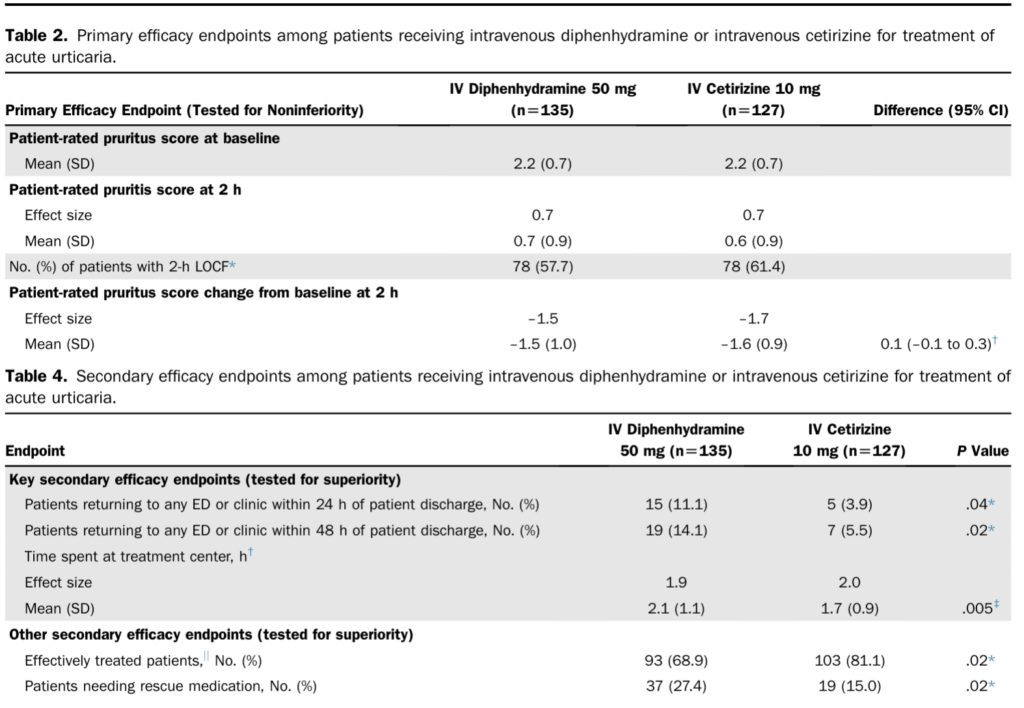
Primary Endpoint:
- The lower bound of the 95% CI for treatment difference did not include –0.5
- The effectiveness of IV cetirizine was determined to be statistically non-inferior.
Key Secondary Endpoints:
- 5 (3.9%) patients in the IV cetirizine group returned to any ED or clinic within 24 hours compared to 15 (11.1%) in the IV diphenhydramine group; P=0.04
- 7 (5.5%) patients in the IV cetirizine group returned to any ED or clinic within 48 hours compared to 19 (14.1%) in the IV diphenhydramine group; P=0.02
- The mean time spent at a treatment center in hours was 1.7 in the IV cetirizine group and 2.1 in the IV diphenhydramine group; P=0.005
Other Secondary Endpoints:
- 19 (15%) patients in the IV cetirizine group received rescue medications compared to 37 (27.4%) patients in the IV diphenhydramine group; P=0.02
- The number of “effectively treated” patients, according to physician assessment, was higher in the IV cetirizine group, with 103 (81.1%) patients compared to 93 (68.9%) patients in the IV diphenhydramine group; P=0.02.
- The change in mean sedation score was 0.2 in the IV cetirizine group compared to 0.7 in the IV diphenhydramine group, P=0.003.
- T5 (3.9%) patients with adverse events were reported in the IV Cetirizine group compared to 18 (3.9%) in the IV diphenhydramine group.
Strengths
- Randomized, double-blinded, multi-center study design increases external validity.
- Appropriate use of noninferiority design
- The study investigates a clinically important, patient-centered question
- The baseline characteristics of study participants in each group were similar
- Treatment drugs were randomized and blinded by the sponsor before delivery to the investigational site
- Drugs visually identical clear aqueous solutions
- A staff member not involved in patient management or outcome assessment was responsible for drawing up medication.
- Health care professional involved in patient management and outcome assessment was completely blinded.
- Investigators performed an intention-to-treat analysis
- Minimal loss to follow up
Limitations
- The availability of IV cetirizine is unclear, and the lack of ubiquitous availability decreases external validity.
- It’s unclear why parenteral medications were chosen over the oral formulation.
- The trial was conducted in a single country which decreases the external validity.
- Many primary and secondary endpoints were subjective
- The pruritus score utilized is not a validated scoring tool
- The non-inferiority margin of –0.5 on a visual analog scale represents approx 16%. The larger the margin, the easier it is for investigators to determine one therapy is noninferior than another
- Patients could still be included in the trial after receiving multiple medications, provided symptoms of urticaria were still present and enough time elapsed
- Receipt of additional medications could confound the results and mask or enhance the effects of the study drug
- It’s unclear how many patients in each cohort received additional medications before enrollment and whether they were balanced across each group
- Numerous conflicts of interest reported
Discussion
- The authors state, “the parenteral route of administration is often preferred to provide a rapid onset of action” (Benjamin 2020). However, according to the American Academy of Allergy, Asthma, and Immunology, “oral antihistamine therapy is equivalent to IV therapy” (Ledford D 2019). While the need for parenteral therapy is apparent in anaphylaxis, it’s more nebulous in minor and moderate non-life-threatening reactions such as urticaria.
- Acute urticaria is often characterized by evanescent phenomena, where lesions come and go randomly in various locations throughout the body until eventual resolution after days. Anecdotally, the persistence of urticaria often has little influence on the decision to discharge a patient from the ED. When considering time spent in the ED, many patients with acute urticaria who are not in anaphylaxis can be treated with oral medicines and expeditiously discharged compared to the time required to place an IV and administer parenteral therapy.
- Noninferiority trials, covered here on REBEL EM, are helpful when the experimental treatment offers a distinct advantage over the standard treatment. In this case, cetirizine may be advantageous as it may have a more favorable side effect profile than diphenhydramine. However, many believe that noninferiority trials are unethical as we are potentially offering patients a worse treatment option. How much worse depends on an often arbitrarily set noninferiority margin—approx 16% in this trial. In a noninferiority trial, we ask if one therapy is not much worse than another.
- It’s unclear what advantage parenteral medications offer compared to oral therapy in this study. Additionally, IV cetirizine costs approximately $320 for a 10 mg dose, or 30 times more than oral cetirizine and 10 times more than IV diphenhydramine.
- There are numerous conflicts of interest. The study was funded by TerSera Therapeutics (makers of IV cetirizine), and multiple authors received consulting fees. TerSera also provided “statistical expertise on study design.” Another pharmaceutical company, Wesley Enterprise Inc., provided medical writing and editorial support. Studies funded by pharmaceutical companies are four times more likely to have favorable results than studies from other sources (Lexchin 2003). Pharmaceutical companies can also design trials to get desired results by conducting a trial against a product known to be inferior.
Authors’ Conclusions: “This study demonstrated that intravenous cetirizine (10 mg) is as effective as intravenous diphenhydramine (50mg) in the treatment of acute urticaria while offering benefits that include less sedation and a lower overall adverse event rate, as well as less time spent at the treatment center, and a lower rate of return to the facility.”
Clinical Bottom Line
This trial was funded, designed, and written with direction from a pharmaceutical company. The data reported are interesting, but we are concerned about significant conflicts of interest. While second-generation antihistamines such as cetirizine may offer some clear advantages over diphenhydramine, we see no clear benefit for parenteral therapy in this study.
Resources:
- Banerji A, Long AA, Camargo CA Jr. Diphenhydramine versus nonsedating antihistamines for acute allergic reactions: a literature review. Allergy Asthma Proc. 2007 Jul-Aug;28(4):418-26. PMID: 17883909.
- Benadryl (diphenhydramine hydrochloride injection,USP) [package insert] New Brunswick , NJ: Johnson & Johnson; 2016.
- Bernstein J, Leslie T. Antihistamines. In: Grammer LC, Greenberger PA, editors. Patterson’s allergic diseases. 8. Philadelphia: Wolters Kluwer Health; 2018. pp. 719–734.
- Church MK, Maurer M, Simons FE, Bindslev-Jensen C, van Cauwenberge P, Bousquet J, Holgate ST, Zuberbier T; Global Allergy and Asthma European Network. Risk of first-generation H(1)-antihistamines: a GA(2)LEN position paper. Allergy. 2010 Apr;65(4):459-66. Epub 2010 Feb 8. PMID: 20146728.
- Clark S, Long AA, Gaeta TJ, Camargo CA Jr. Multicenter study of emergency department visits for insect sting allergies. J Allergy Clin Immunol. 2005 Sep;116(3):643-9. PMID: 16159637.
- Clark S, Bock SA, Gaeta TJ, Brenner BE, Cydulka RK, Camargo CA; Multicenter Airway Research Collaboration-8 Investigators. Multicenter study of emergency department visits for food allergies. J Allergy Clin Immunol. 2004 Feb;113(2):347-52. PMID: 14767453.
- Clark, S. et al. (2006) “Management of acute allergic reactions and anaphylaxis in the Emergency Department between 1993-2003,” Journal of Allergy and Clinical Immunology, 117(2).
- Ledford, D. (2019) Substitute for diphenhydramine, American Academy of Allergy Asthma & Immunology. Available at: https://www.aaaai.org/Allergist-Resources/Ask-the-Expert/Answers/Old-Ask-the-Experts/diphenhydraminess (Accessed: December 13, 2023).
- Monroe EW, Jones HE. Urticaria. An updated review. Arch Dermatol. 1977;113:80-90.
- Schaefer P. Acute and Chronic Urticaria: Evaluation and Treatment. Am Fam Physician. 2017 Jun 1;95(11):717-724. PMID: 28671445.
- Zuberbier, Torsten, et al. “Definition, Classification, and Routine Diagnosis of Urticaria: A Consensus report11this Consensus Report Is the Result of a Panel Discussion during the International Clinically Oriented ESDR Symposium ‘Urticaria 2000’.” Journal of Investigative Dermatology Symposium Proceedings, vol. 6, no. 2, 2001, pp. 123–127.,
Guest Post By:

Nadia Adside, MD
PGY-2, Emergency Medicine Resident
Vassar Brothers Hospital, Poughkeepsie, New York
E-mail: nadia.adside@nuvancehealth.org


Marco Propersi, DO FAAEM
Vice-Chair, Emergency Medicine
Vassar Brothers Hospital, Poughkeepsie, New York
Associate Editor, REBEL EM Blog
Twitter: @marco_propersi
Post-Peer Reviewed By: Salim R. Rezaie, MD (Twitter: @srrezaie)
The post Cetirizine Vs Diphenhydramine For the Treatment of Acute Urticaria in the ED appeared first on REBEL EM - Emergency Medicine Blog.
