 Background: We have covered the two previous RCTs on remdesivir on REBEL EM (RCT #1 and RCT #2). In the first trial by Wang et al [2], there was no statically significant improvement in clinical outcomes, but, there were trends toward shorter duration of illness. In the ACTT-1 preliminary report [3], despite all the methodological issues, there was a 4 day decrease in clinical improvement (although not in patients requiring HFNC/NIV/IMV/ECMO). Neither trial was perfect, however in the middle of pandemic, a several day decrease in recovery time may be beneficial in reducing hospital crowding if the difference holds true in subsequent studies and if the correct target population is known. We now have our 3rd RCT on remdesivir [1], just published in the NEJM comparing 5 days vs 10 days of remdesivir in patients with severe COVID-19.
Background: We have covered the two previous RCTs on remdesivir on REBEL EM (RCT #1 and RCT #2). In the first trial by Wang et al [2], there was no statically significant improvement in clinical outcomes, but, there were trends toward shorter duration of illness. In the ACTT-1 preliminary report [3], despite all the methodological issues, there was a 4 day decrease in clinical improvement (although not in patients requiring HFNC/NIV/IMV/ECMO). Neither trial was perfect, however in the middle of pandemic, a several day decrease in recovery time may be beneficial in reducing hospital crowding if the difference holds true in subsequent studies and if the correct target population is known. We now have our 3rd RCT on remdesivir [1], just published in the NEJM comparing 5 days vs 10 days of remdesivir in patients with severe COVID-19.
Paper: Goldman JD et al. Remdesivir for 5 or 10 Days in Patients with Severe COVID-19. NEJM 2020. [Epub Ahead of Print]
Clinical Question: In patients with COVID-19 pneumonia is a 5 day course as effective as a 10 day course of remdesivir in improving clinical course at 14 days?
What They Did:
- Multicenter, phase 3, randomized, open-label, phase 3 trial of hospitalized patients with COVID-19, O2 saturation of ≤94% on room air, and radiologic evidence of pneumonia
- Patients enrolled from 55 hospitals globally
- Randomized in 1:1 ratio to remdesivir IV for 5 days vs 10 days
- All patients received 200mg remdesivir on day 1 and then 100mg once daily on subsequent days
Outcomes:
- Primary: Clinical status on day 14, assessed on a 7-point ordinal scale
- Secondary: Proportion of patients with adverse events
Inclusion:
- ≥12 years of age
- Hospitalized
- Confirmed SARS-CoV-2 infection
- Within 4d before randomization
- Radiographic evidence of pulmonary infiltrates
- O2 saturation <94% on room air or receiving supplemental O2
Exclusion:
- Invasive mechanical ventilation
- ECMO
- Multiorgan failure
- AST/ALT >5x upper limit of normal range
- eGFR <50mL/min (Excluded patients with kidney disease)
- Receiving concurrent treatment (within 24hrs before the start of trial treatment) with other agents with activity against COVID-19
Results:
- 397 patients randomized and began treatment
- Clinical improvement of ≥2 points on the ordinal scale by day 14:
- 10d: 54%
- 5d: 64%
- In the unadjusted analysis for baseline clinical status the OR was 0.67 (95% CI 0.46 to 0.97), favoring the 5d group, but in the adjusted analysis this was no longer statistically significant (OR 0.75; 95% CI 0.51 to 1.12)
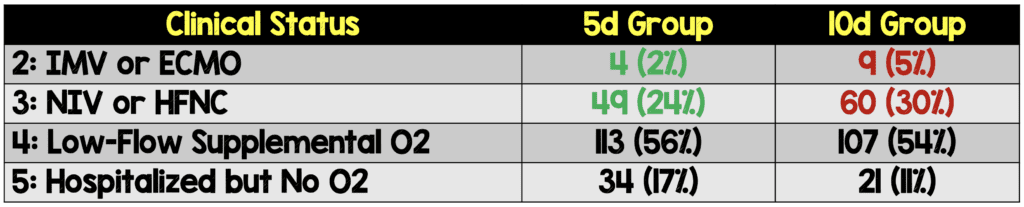
- Numerically it seemed that the 5d group fared better than the 10d group:
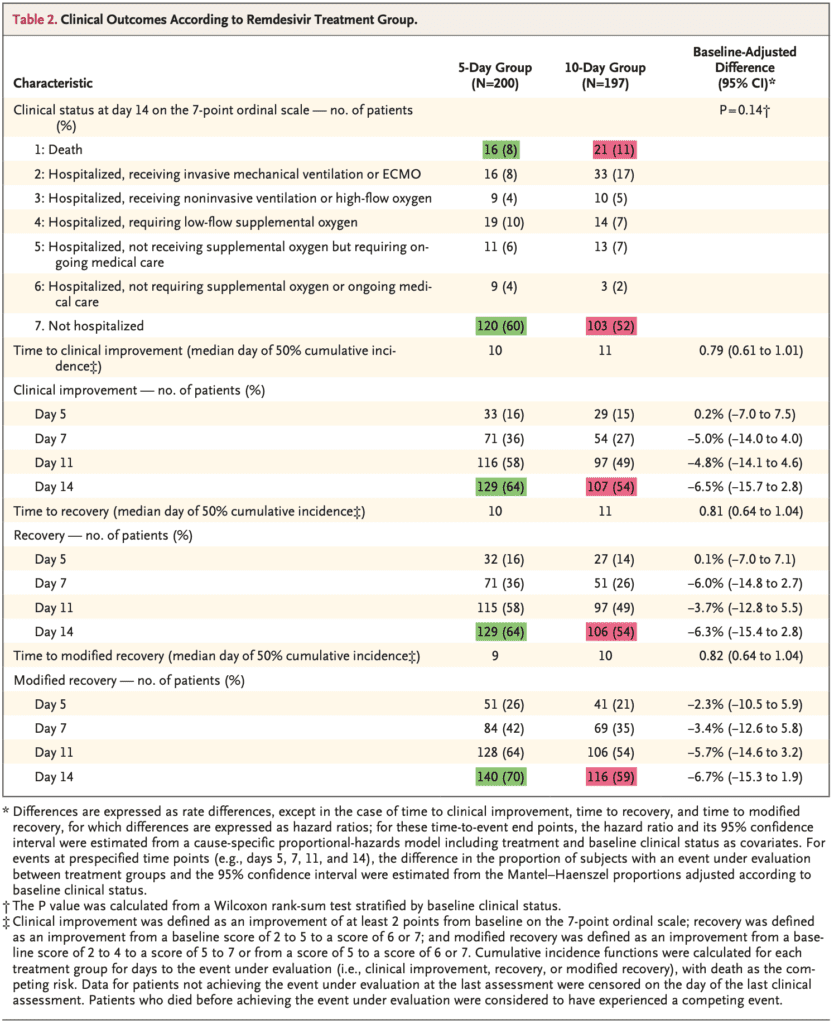
- The percentages of patients experiencing adverse events was similar between groups, however there were slightly more serious adverse events in the 10d vs 5d groups (35% vs 21%). The most common adverse events:
- Nausea: 9%
- Worsening respiratory failure: 8%
- Elevated ALT: 7%
- Constipation: 7%
- Patients in the 10d cohort had more kidney problems compared to the 5d cohort:
- Decreased Creatinine Clearance (Grade 4: 12% vs 3%)
- Increased Acute Kidney Injury (8% vs 4%)
Strengths:
- Multicenter, randomized clinical trial
- Asks a clinically important question, as remdesivir is expensive and in limited supply
Limitations:
- Patients in the 10-day arm were sicker at baseline compared to the 5-day arm
- NO placebo group, therefore it is unclear if remdesivir performs better than placebo
- Trial designed and conducted by Gilead Sciences, the maker of remdesivir. They also collected the data, monitored the conduct of the trial, performed statistical analyses, and wrote the manuscript. This does not negate the findings of the study, however if you are the company making a medication and want to prove it is effective, you would expect a slam dunk positive study, which I would argue this trial is not
- Results cannot be extrapolated to patients receiving mechanical ventilation or ECMO
Discussion:
- The protocol was amended on March 15th, 2020 (after the beginning of enrollment but before any results were available)
- Lower age limit for eligibility reduced from 18 to 12 years
- Requirement of an axillary temperature of at least 36.6C at screening eliminated
- Primary efficacy assessment (proportion of patients with normalization of temperature at day 14 was changed to assessment of clinical status on a 7-point ordinal scale on day 14)
- There was only one real secondary outcome in this study. This might be the shortest list of secondary outcomes, I have ever seen in a study
- Groups were not balanced at baseline clinically, as there was a greater proportion of patients in the two highest disease severity groups (IMV, HFNC) in the 10d vs 5d groups. In a randomized clinical trial, you would expect groups to be balanced, however sometimes it can happen, just by chance alone, that the groups are unbalanced
- Patients in the 10d cohort often didn’t complete a full 10 days of treatment (i.e. 44% of group) due to discharge (35%), adverse events (11%), or death (6%). This is compared to an 86% completion rate in the 5d cohort. Something to consider is that if less ill patients are likely to be discharged then that means the remaining patients staying in the hospital are sicker. This may be one of the reasons we see more adverse events in the 10d group compared to the 5d group, but regardless it appears clinical improvement is essentially the same between the two groups.
- No placebo group is a big issue with this trial as we don’t know if clinical improvement would occur at the same rate in a disease that is self-limited (i.e. without placebo we cannot test the efficacy of remdesivir)
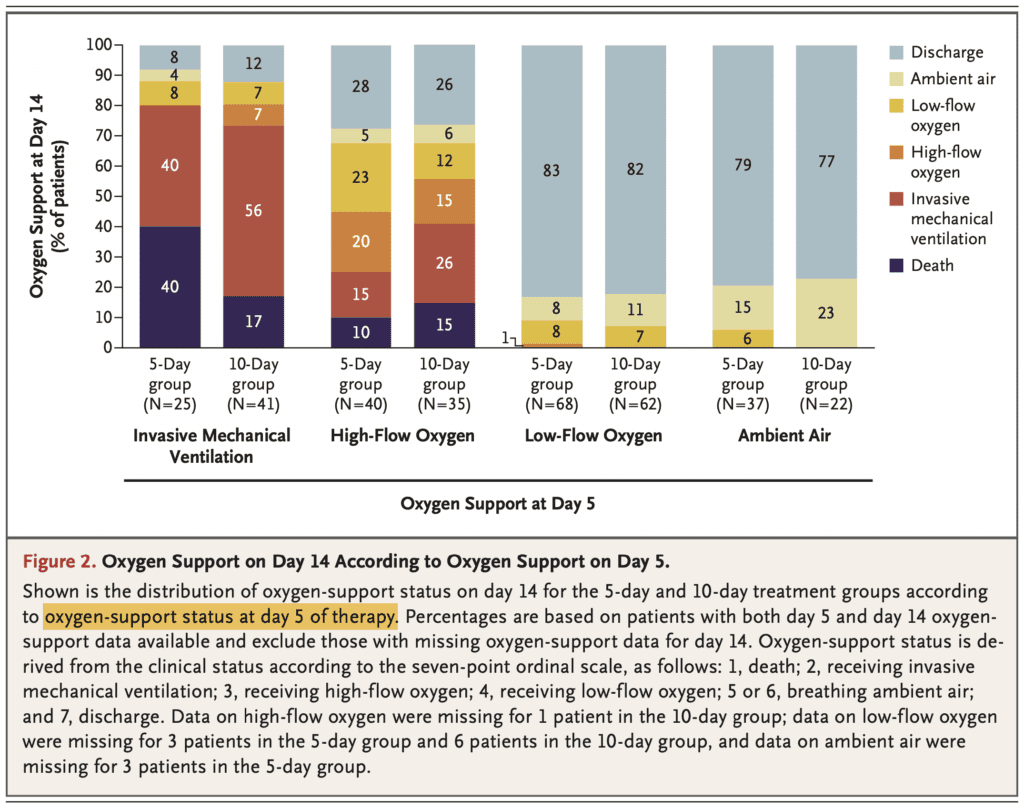
- In a post-hoc analysis the authors compared requirement of oxygen support at day 14 of the 5-day vs 10-day cohorts, to look for differences in subgroups.
- When more analyses are performed, there is a higher likelihood of a positive outcome from chance alone. It is, therefore, unclear if these results are true or just statistical noise.
- Also, confusing to me is that the subgroups were based on patient’s clinical status on day 5 of therapy and not at treatment initiation (i.e. it doesn’t make sense to start treatment for 5 days then do a subgroup analysis).
Author Conclusion: “In patients with severe COVID-19 not requiring mechanical ventilation, our trial did not show a significant difference between a 5-day course and a 10-day course of remdesivir. With no placebo control, however the magnitude of benefit cannot be determined.”
Clinical Take Home Point: The results of this study demonstrate that a 5-day course of remdesivir results in better clinical outcomes than a 10-day course in hospitalized patients with COVID-19 pneumonia, not on mechanical ventilation. It is important to note that the study contains serious methodological flaws, does not show that remdesivir is better than placebo and attempts to hide worse outcomes of the longer course with statistical manipulation .
References:
- Goldman JD et al. Remdesivir for 5 or 10 Days in Patients with Severe COVID-19. NEJM 2020. [Epub Ahead of Print]
- Wang Y et al. Remdesivir in Adults with Severe COVID-19: A Randomized, Double-Blind, Placebo-Controlled, Multicentre Trial. Lancet 2020. [Epub Ahead of Print]
- Beigel JH et al. Remdesivir for the Treatment of COVID-19 – Preliminary Report. NEJM 2020. [Epub Ahead of Print]
For More Thoughts on This Topic Checkout:
- REBEL EM: COVID-19 – Two More Trials Just Published on Remdesivir
- REBEL EM: Remdesivir ACTT-1 – Adaptive COVID-19 Treatment Trial Part 1
- PulmCrit: Remdesivir 5-Day vs 10-Day Trial Raises Some Red Flags?
- FOAMCast: COVID-19 – Remdesivir 5 vs 10 Days
Post Peer Reviewed By: Anand Swaminathan, MD (Twitter: @EMSwami)
The post COVID-19: Remdesivir RCT #3 (5d vs 10d) appeared first on REBEL EM - Emergency Medicine Blog.