
 Background: Medicine by press release has been an unfortunate reality of the COVID-19 pandemic. This is not the standard many physicians would use for medical decision making, but one many of us have had to face; what do I do with the information that is presented with no transparency in the data behind the statements? Despite all the debate and controversy only two treatments for hospitalized patients, have been shown to improve mortality: corticosteroids and IL-6 receptor antagonists (when combined with steroids). Two more treatment options that have been debated throughout the pandemic include convalescent plasma therapy and anticoagulation. We now have two pre-prints that shed some more light on both of these topics.
Background: Medicine by press release has been an unfortunate reality of the COVID-19 pandemic. This is not the standard many physicians would use for medical decision making, but one many of us have had to face; what do I do with the information that is presented with no transparency in the data behind the statements? Despite all the debate and controversy only two treatments for hospitalized patients, have been shown to improve mortality: corticosteroids and IL-6 receptor antagonists (when combined with steroids). Two more treatment options that have been debated throughout the pandemic include convalescent plasma therapy and anticoagulation. We now have two pre-prints that shed some more light on both of these topics.
Convalescent Plasma Therapy
Convalescent plasma therapy has been administered tens of thousands of times in the US (including by me) to treat hospitalized COVID patients despite a lack of strong evidence. Most of the evidence thus far has been in the form of observational trials, not randomized clinical trials. The US Expanded Access Program, was a multicenter, open-label trial of over 47,000 patients hospitalized with COVID-19. This trial showed that earlier transfusion (≤3d vs ≥4d), was associated with improved 7d and 30d mortality. Additionally, higher titer compared to lower titer plasma was also associated with improved mortality. However, without a standard care arm there was no way to know if convalescent plasma therapy would offer anything over usual care. More recently, smaller RCTs have emerged that demonstrate no benefit for CPT (PlasmAr Trial).
Paper: The RECOVERY Collaborative Group. Convalescent Plasma in Patients Admitted to Hospital with COVID-19 (RECOVERY): A Randomised, Controlled, Open-Label, Platform Trial. medRxiv Preprint 2021. [Link is HERE]
Clinical Question: What is the safety and efficacy of convalescent plasma in patients admitted to the hospital with COVID-19 compared to usual care?
What They Did:
- Randomised Evaluation of COVID-19 Therapy (RECOVERY)
- Randomized, controlled, open-label, adaptive platform trial performed in the UK
- Patients randomized 1:1:1 to:
- Convalescent Plasma (High titer) + Usual care
- Only plasma donations with a sample to cut-off (S/CO) ratio of ≥6 on the EUROIMMUN IgG ELISA test targeting the spike glycoprotein were supplied for the RECOVERY trial
- The US FDA has determined that convalescent plasma with a EUROIMMUN S/CO of ≥3.5 qualifies as high titer
- Two units (275mL +/- 75mL) were give IV: 1st dose as soon as possible after randomization and the 2nd dose the following day and at least 12hrs after the 1st dose
- REGN-COV2 + Usual care (Ongoing and not reported)
- Usual care
- Convalescent Plasma (High titer) + Usual care
- As a platform trial patients could also be simultaneously randomized to other treatment options:
- Hydroxychloroquine
- Dexamethasone
- Azithromycin
- Lopinavir-Ritonavir
- Aspirin
- Colchicine
- Tocilizumab
- Several of these treatment arms were added or removed from the protocol over the period that convalescent plasma was evaluated
Outcomes:
- Primary: 28d Mortality
-
Secondary:
- Time to discharge from hospital
- Among patients not receiving invasive mechanical ventilation at randomization:
- Subsequent receipt of invasive mechanical ventilation (including ECMO) or death
-
Safety:
- Transfusion related adverse events at 72hrs following randomization
- Cause-specific mortality
- Major cardiac arrhythmia
Inclusion:
- Hospitalized patients
- Clinically suspected or laboratory-confirmed SARS-CoV-2 infection
- No medical history that might, in the opinion of the clinician, put them at significant risk if they were to participate in the trial
- Written informed consent obtained
Exclusion:
- Patient declined convalescent plasma
- Convalescent plasma unavailable at trial site
- In the opinion of the clinician caring for the patient contraindicated (known moderate or severe allergy to blood components or unwilling to receive a blood product)
Results:
- Patients Included in RECOVERY Trial
- 13,127 (81%) of 16,287 patients eligible to be randomized
- Convalescent Plasma + Usual Care: 5,795
- 4,675 (81%) received 2 units
- 671 (12%) received 1 unit
- 449 (8%) received 0 units
- Usual Care Alone: 5,763
- REGN-COV2: Remainder of patients (Not reported here)
- Convalescent Plasma + Usual Care: 5,795
- Median time from symptom onset = 9d (Range 6 to 12d)
- Receiving invasive mechanical ventilation = 617 (5%) of patients
- Receiving O2 only (with or without NIV) = 87% of patients
- Receiving no O2 therapy = 897 (8%) of patients
- Receiving corticosteroids = 92% of patients
- 9,385 (81%) of patients had baseline serology results available
- SARS-CoV-2 Antibody Seropositive = 62%
-
28d Mortality (Primary Outcome):
- Convalescent Plasma: 24%
- Usual Care: 24%
- RR 1.00; 95% CI 0.93 to 1.07; p = 0.93
- RR was similar in all prespecified subgroups of patients including those without detectable SARS-CoV-2 antibodies at randomization
- Discharged from Hospital Alive at 28d:
- Convalescent Plasma: 66%
- Usual Care: 67%
- RR 0.98; 95% CI 0.94 to 1.03; p = 0.50
- Median time to discharge was 11d in both groups
- Among Patients NOT on Invasive Mechanical Ventilation at Baseline:
- Composite Endpoint of Progression to Invasive Mechanical Ventilation or Death
- Convalescent Plasma: 28%
- Usual Care: 29%
- RR 0.99; 95% CI 0.93 to 1.05; p = 0.79
- There was some heterogeneity based on SARS-CoV-2 antibody test result with slightly more favorable outcomes with convalescent plasma seen among seronegative vs seropositive patients
- No difference in use of ventilation, successful cessation of invasive mechanical ventilation, or progression to use of renal replacement therapy
- Severe Allergic Reactions within 1st 72hrs After Randomization:
- Convalescent Plasma: 16 patients
- Usual Care: 2 patients
- Composite Endpoint of Progression to Invasive Mechanical Ventilation or Death
- 13,127 (81%) of 16,287 patients eligible to be randomized
Strengths:
- Largest randomized clinical trial on the topic of convalescent plasma therapy
- Asks a clinically important question
- Baseline SARS-CoV-2 serostatus for most participants were determined at the time of randomization
- Included safety outcomes to blood component transfusions
- Used an intention to treat analysis which represents what actually happens in clinical practice
- Had pre-specified subgroups that were evaluated on the primary outcome adding further validity to the results (included presence of anti-SARS-CoV-2 antibody)
- Performed a post-hoc analysis fo see if there was a difference in the effectiveness of convalescent plasma before and after the emergence of the B117 variant
- Use of corticosteroids and remdesivir following randomization was similar between groups
- Used high-titer convalescent plasma as there has been previous evidence that low titers could explain negative results from previous randomized trials
Limitations:
- There was an imbalance of baseline serology samples missing in the usual care arm. Authors suspect this is a mistaken belief that a serology sample was only required in patients allocated to convalescent plasma
- Slightly fewer patients received tocilizumab or sarilumab in the convalescent plasma group vs usual care (8% vs 10%)
- Open-label trial in which participants and clinicians knew exactly which patients were getting treatment. This could affect subjective outcomes
- Did not include outpatients, preventing any conclusions in these patients
- 62% of patients that were tested had antibodies prior to CPT. Days from onset to therapy was 9. It is possible that CPT would have more value if given early before patients have an antibody response
Discussion:
- Convalescent plasma from individuals infected prior to the emergence of B117 (UK variant) show a modest reduction in ability to neutralize B117 compared with earlier SARS-CoV-2 variants. The clinical significance of this reduced in vitro neutralization is not known at this time
- Sample size calculations could not be estimated when the trail was being planned at the start of the COVID-19 pandemic. During the trial, external data suggested that benefits of antibody-based therapies may be greater among those patients who had not raised an adequate antibody response of their own. While still blind to the results of the trial, the RECOVERY steering committee determined that the trial should enroll enough patients to provide at least a 90% power to detect a proportional reduction in 28d mortality of 1/5th among those patients with and separately without detectable SARS-CoV-2 antibodies at randomization
- An interesting point brought up in the paper is that 28d mortality was higher among patients who were seronegative at randomization, BUT the proportional effect of allocation to convalescent plasma on 28d mortality was similar among seropositive patients:
- Seropositive: 19% vs 18%; RR 1.05; 95% CI 0.93 to 1.19
- Seronegative: 32% vs 34%; RR 0.94; 95% CI 0.84 to 1.06
- Taking all the RCTs available together, there is no improvement in mortality with convalescent plasma therapy (Mortality RR 0.99; 95% CI 0.92 to 1.06; p = 0.77)
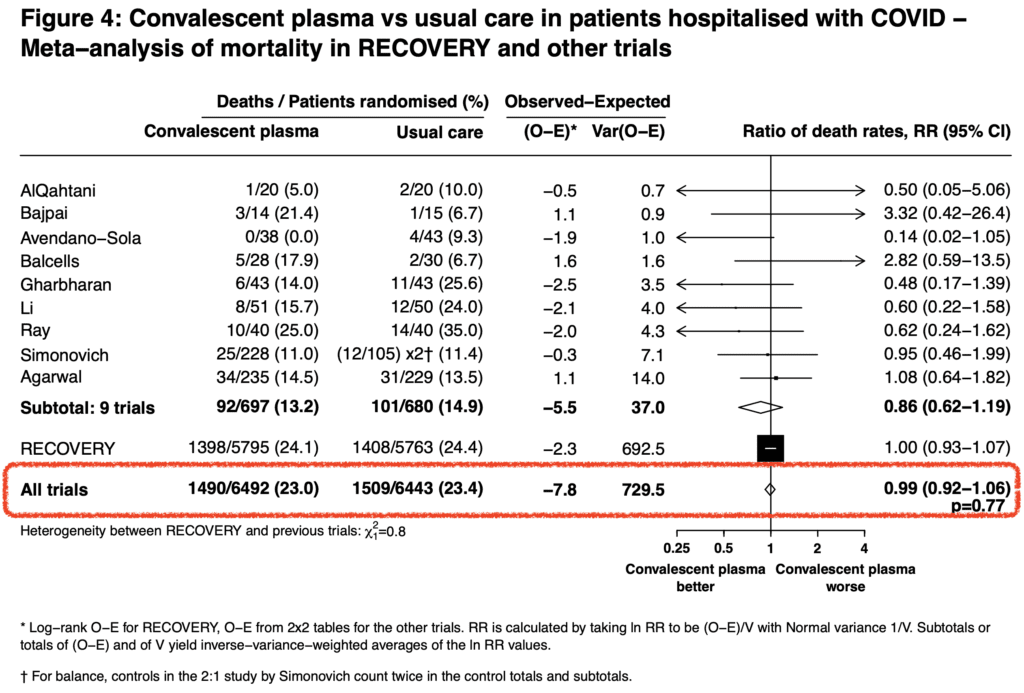
- In this trial the lack of anti-SARS-CoV-2 antibodies prior to transfusion with convalescent plasma was 38% of patients. And although they had a higher mortality risk than seropositive patients, there was no observed survival benefit from convalescent plasma therapy
- The leading thought with convalescent plasma therapy is that it should be most effective in the early stages of infection, when viral replication dominates. The authors of the RECOVERY trial did not find a benefit when patients were stratified by time since onset of illness in either the main analysis or any of the exploratory analyses (IMPORTANT POINT: These were only hospitalized patients. It is unclear from this data if convalescent plasma may be of benefit if given early after SARS-CoV-2 infection as outpatients)
- While B117 variant has changes in the spike glycoprotein that could modify the effect of convalescent plasma therapy not having antibodies to this strain, in this trial there was no identification of any evidence of a differential effect whether convalescent plasma given was prior to or after the emergence of the B117 variant
Author Conclusion: “Among patients hospitalized with COVID-19, high-titre convalescent plasma did not improve survival or other prespecified clinical outcomes.”
Clinical Take Home Point: Convalescent plasma therapy should no longer be used in hospitalized patients with COVID-19. In hospitalized patients with COVID-19 there is no high-quality data to support the use of convalescent plasma therapy. The use of convalescent plasma therapy has been an appealing treatment option but, we now have a well done randomized clinical trial that shows no improvement in survival or other prespecified clinical outcomes regardless of the status of anti-SARS-CoV-2 antibodies.
Anticoagulation
Early, observational trials found an association between COVID-19 and hypercoagulability/thrombosis. Even when compared to H1N1, COVID-19 was shown to have 9x more alveolar capillary microthrombi. Additionally, being in the ICU itself is known to increase the risk of thrombotic events. Throughout the pandemic, there has been huge debate about which anticoagulation regimen is best in critically ill patients with COVID-19: prophylactic, intermediate, or full dose. Even more unclear, based on available evidence, is what is the safety of these strategies and do they improve patient-oriented outcomes compared to usual care?

Paper: The REMAP-CAP, ACTIV-4a, and ATTACC Investigators. Therapeutic Anticoagulation in Critically Ill Patients with COVID-19 – Preliminary Report. medRxiv Preprint 2021. [Link is HERE]
Clinical Question: Does therapeutic anticoagulation improve outcomes in critically ill patients with COVID-19 compared to other anticoagulation regimens?
What They Did:
- Open-label, adaptive, multiplatform, randomized, clinical trial
- Patients randomized to:
- Therapeutic anticoagulation with heparin (unfractionated or low molecular weight)
- Pharmacological thromboprophylaxis as per local usual care
Outcomes:
-
Primary: Organ support free days (OSFDs)
- Ordinal scale composed of survival to hospital discharge, in survivors, the number of days free of organ support to day 21
- Death in hospital through day 21 was assigned worst outcome = -1
- A higher value indicates a better outcome
-
Secondary:
- Survival to day 90
- Major thrombotic events or death (A composite of MI, PE, ischemic stroke, systemic arterial embolism, and in-hospital death) through 28d (ACTIV-4a, ATTACC) or through to hospital discharge (REMAP-CAP)
-
Safety:
- Major bleeding
- Heparin-induced thrombocytopenia
Inclusion:
- Severe COVID-19 (Requirement for organ support with HFNC ≥20L/min, NIV, IMV, vasopressors or inotropes)
- Confirmed COVID-19 infection
Exclusion:
- Admitted to ICU with COVID-19 for >48hrs (REMAP-CAP)
- Admitted to the hospital with COVID-19 for >72hrs (ACTIV-4a, ATTACC)
- Imminent risk of death without an ongoing commitment to full organ support
- High risk of bleeding
- Receiving dual antiplatelet therapy
- Separate clinical indication for therapeutic anticoagulation
- History of heparin sensitivity (i.e. HIT)
Results:
- 1,089 patients were included in the primary outcome analysis
- Of the non-therapeutic anticoagulation patients:
- Standard low dose thromboprophylaxis = 41% of participants
- Enhanced intermediate dose thromboprophylaxis = 51% of participants
- Median d-dimer ≈850ng/mL
- Of the non-therapeutic anticoagulation patients:
- Median Organ Support Free Days:
- Therapeutic AC: 3d (-1 to 16)
- Thromboprophylaxis AC: 5d (-1 to 16)
- aOR 0.87; 95% CI 0.70 to 1.08
- In-Hospital Survival:
- Therapeutic AC: 64.3%
- Thromboprophylaxis AC: 65.3%
- aOR 0.88; 95% CI 0.67 to 1.16
- Major Thrombotic Events:
- Therapeutic AC: 5.7%
- Thromboprophylaxis: 10.3%
- Major Bleeding:
- Therapeutic AC: 3.1%
- Thromboprophylaxis AC: 2.4%
Strengths:
- Lead investigators of three international adaptive platform trials (REMAP-CAP, ACTIV-4a, and ATTACC) aligned their trial design, eligibility criteria, interventions, and outcome measures to execute a single RCT
- Largest RCT on this topic to date
- Asks a clinically important question
- Uses treatments that are familiar and widely accessible
- Analysis was pre-specified and used a Bayesian framework during interim analyses
- Groups fairly well balanced with same number of patients receiving remdesivir, corticosteroids, and tocilizumab between groups
Limitations:
- Open-label trial in which clinicians knew which patients were getting what. This can bias subjective outcomes
- Both therapeutic and prophylactic anticoagulation were administered according to local site protocols. This makes evaluation of this trials results messy. For example, standard low dose and enhanced intermediate dose thromboprophylaxis were considered the same thing
- The median d-dimer level was ≈850ng/mL in this trial. Only 47% of patients had a d-dimer level ≥2x the upper limit of normal. This does not allow any conclusions in patients with higher d-dimer levels
Discussion:
- Majority of patients enrolled from the REMAP-CAP platform (n = 987; 84%)
- Important to note that in this trial although therapeutic anticoagulation resulted in a numerical decrease in major thrombotic events, it did not improve organ support-free days or survival to hospital discharge
- One thing to consider is that in patients with severe disease, it may just simply be too late in the process to modify the already established disease pathology
- You have to dig through the supplementary material to find exactly what anticoagulation strategy was given in what percentage of patients and it’s a hot mess. As mentioned in the results above, the thromboprophylaxis group is almost an even split between low dose thromboprophylaxis and intermediate dose thromboprophylaxis. To make it worse, only 78% of patients in the therapeutic anticoagulation group actually got therapeutic dose anticoagulation (see table below)
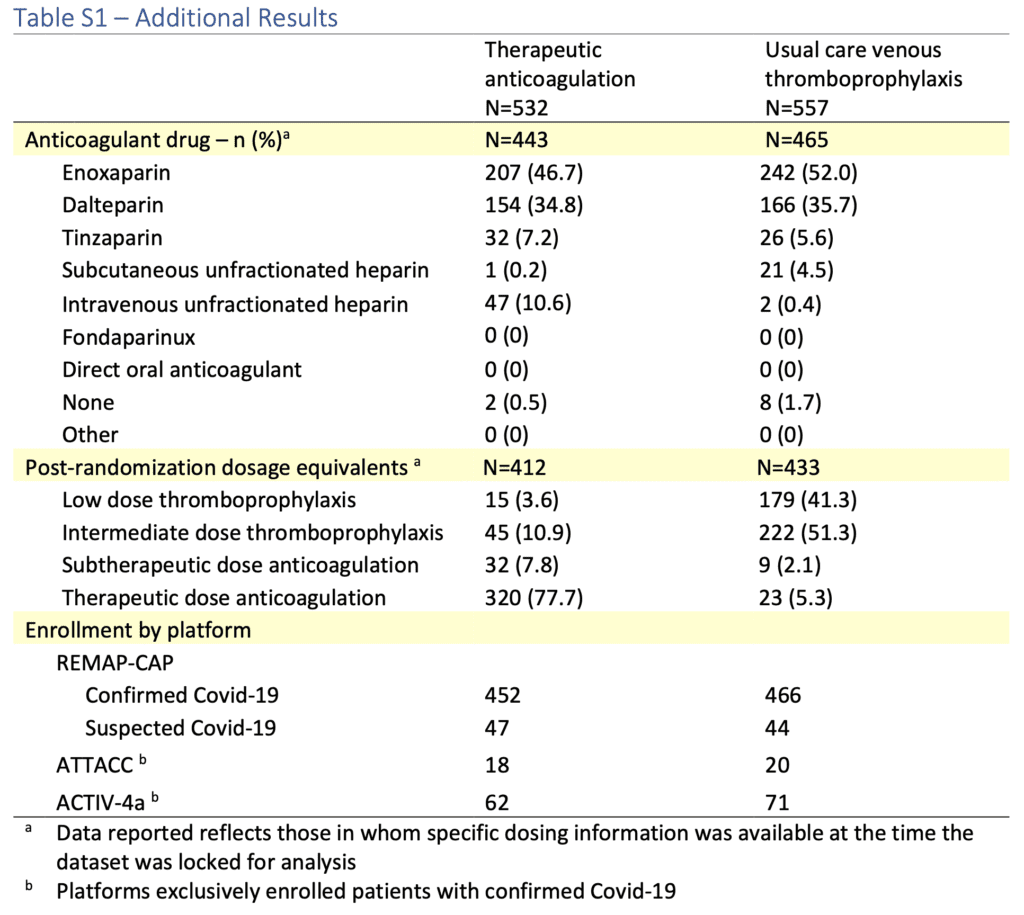
Author Conclusion: “In patients with severe COVID-19, therapeutic anticoagulation did not improve hospital survival or days free of organ support compared to usual care pharmacological thromboprophylaxis.”
Clinical Take Home Points:
- This is the best RCT data on the topic of anticoagulation and it demonstrates no patient oriented benefit of therapeutic anticoagulation in critically ill COVID-19 patients. However, this data is inadequate in guiding management because
- I commend the 3 platforms for combining efforts in trying to get us closer to an answer on the topic of anticoagulation in severely ill patients with COVID-19
- Unfortunately, due to the multiple dosing regimens used throughout the study, it is unclear what conclusion to draw from this trial
- Additionally, the average d-dimer level was ≈850ng/mL making it impossible to say if we would see a greater benefit from therapeutic anticoagulation in patients with higher d-dimer values
- Despite the shortcomings of the trial, one thing is clear, therapeutic anticoagulation decreased thrombotic events without increasing bleeding. This leaves the door open for an individualized decision making on which anticoagulation strategy is best
References:
- The RECOVERY Collaborative Group. Convalescent Plasma in Patients Admitted to Hospital with COVID-19 (RECOVERY): A Randomised, Controlled, Open-Label, Platform Trial. medRxiv Preprint 2021. [Link is HERE]
- The REMAP-CAP, ACTIV-4a, and ATTACC Investigators. Therapeutic Anticoagulation in Critically Ill Patients with COVID-19 – Preliminary Report. medRxiv Preprint 2021. [Link is HERE]
For More Thoughts on This Topic Checkout:
- PulmCrit: High-Titer Convalescent Plasma Fails Again for Hospitalized COVID Patients
- PulmCrit: Therapeutic Anticoagulation for COVID ICU Patients – Is the Heparin Vial Half Empty, or Half Full?
Post Peer Reviewed By: Anand Swaminathan, MD (Twitter: @EMSwami)
The post COVID-19 Update: Convalescent Plasma Therapy & Anticoagulation appeared first on REBEL EM - Emergency Medicine Blog.
