This publication has now been retracted by Lancet (June 4, 2020)
Background: There is no conclusive evidence that chloroquine, or its derivative hydroxychloroquine, with or without a second-generation macrolide is effective in COVID-19 treatment or prophylaxis. Laboratory studies have shown antiviral and immunomodulatory properties in vitro. The small retrospective observational trials thus far have had mixed results in efficacy. However, these medications are well known to have cardiovascular adverse effects via QT interval prolongation leading to ventricular arrhythmias. Despite the potential harms and the absence of convincing data to support treatment with these drugs, they are widely prescribed to COVID-19 patients.
This publication has now been retracted by Lancet (June 4, 2020)
Due to concerns with respect of the data and analyses conducted by Surgisphere Corporation, an independent third party peer review was launched to evaluate the origination of the database and to confirm the completeness of the database. Surgisphere would not transfer the full dataset, as this would violate client agreements and confidentiality. Therefore an independent peer review was not possible and the paper has been retracted.
NEJM (ACEI/ARB) & Lancet (HCQ/CQ) publications via Mehra et al now retracted
NEJM: https://t.co/ZVZnHKB7ox
Lancet: https://t.co/CFtGNk5mgB#COVID19 #COVID19FOAM #LancetNEJMGate pic.twitter.com/bB0LXDcDmO— Salim R. Rezaie, MD (@srrezaie) June 4, 2020
Paper: Mehra MR et al. Hydroxychloroquine or Chloroquine With or Without a Macrolide for Treatment of COVID-19: A Multinational Registry Analysis. Lancet 2020. [Epub Ahead of Print]
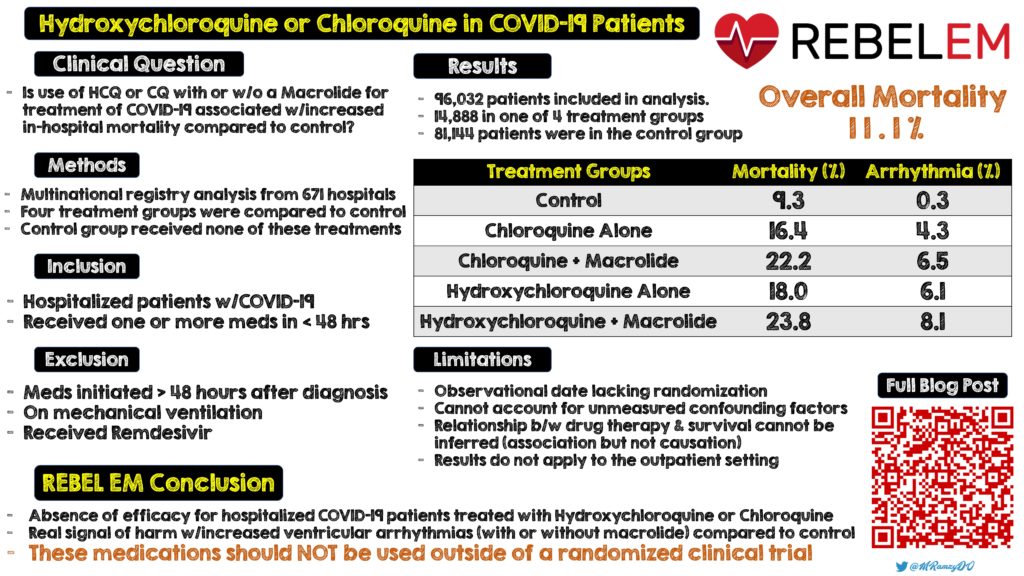
Clinical Question: Is the use of hydroxychloroquine or chloroquine (used with or without macrolides) for treatment of COVID-19 associated with increased in-hospital mortality as compared to control patients not receiving these medications?
What They Did:
- Multinational registry analysis from 671 hospitals across six continents using hydroxychloroquine or chloroquine with or without a macrolide for the treatment of COVID-19
- Patients who received one of the treatments of interest ≤48hrs of diagnosis were included in one of 4 treatment groups:
- Chloroquine Alone (CQ)
- Chloroquine + Macrolide (CQM)
- Hydroxychloroquine Alone (HCQ)
- Hydroxychloroquine + Macrolide (HCQM)
- Patients who received none of these treatments formed the control group (Control)
Outcomes:
- Primary: In-hospital mortality
-
Secondary:
- Occurrence of de-novo ventricular arrhythmias (non-sustained or sustained VT or VF)
- Progression to mechanical ventilation
- ICU LOS
Inclusion:
- Hospitalized patients with COVID-19
- Either hospital discharge or death during hospitalization recorded
- Receiving one or more of the test medications ≤48hrs
Exclusion:
- Treatment initiated >48hrs after diagnosis
- On mechanical ventilation
- Received remdesivir
Results:
- 96,032 patients included in analysis
- 14,888 patients in one of 4 treatment groups
- 81,144 patients were in the control group
- Baseline severity of patients was overall pretty low (≈10% requiring O2)
- Overall mortality rate of 11.1%
- Median time form hospitalization to diagnosis of COVID-19 was 2 days (Range: 1 to 4d)
-
In-Hospital Mortality:
- CQ: 16.4%
- CQM: 22.2%
- HCQ: 18.0%
- HCQM: 23.8%
- Control: 9.3%
- All independently associated with increased risk of in-hospital mortality compared to control
-
De-Novo Ventricular Arrhythmia During Hospitalization:
- CQ: 4.3%
- CQM: 6.5%
- HCQ: 6.1%
- HCQM: 8.1%
- Control: 0.3%
- Also, all independently associated with increased risk of de-novo ventricular arrhythmia during hospitalization compared to control
Strengths:
- Largest, most comprehensive data set to date evaluating the use of hydroxychloroquine and chloroquine (with or without a macrolide)
- Evaluated patients from across multiple geographic regions which increases generalizability and is one of the most robust real-world pieces of evidence to date on these medications
- Used propensity score matching analysis for each group individually compared with control group to approximate demographics, comorbidities, and disease severity
- Hard, objective, patient centered outcome
- Patients fairly well balanced between all groups
- Performed additional analyses to evaluate the robustness of the initial results
- All included patients completed their hospital course (discharged or died)
Limitations:
- Observational data, which cannot account for unmeasured confounding factors
- Due to lack of randomization, unable to control for other parts of management, minimal discussion of what was standard care
- As this is an observational trial a causal relationship between drug therapy and survival cannot be inferred (i.e. association but not causation)
- Results do not apply to outpatient setting
Discussion:
- Overall, most common concurrent use of antivirals in this study were: lopinavir/ritonavir (31.6%), ribavirin (20.3%), and oseltamivir (13.1%)
- Patients overall weren’t that sick (>80% with a SOFA score of 1 and only 10% with an oxygen saturation of <94%)
Author Conclusion: “We were unable to confirm a benefit of hydroxychloroquine or chloroquine, when used alone or with a macrolide, on in-hospital outcomes for COVID-19. Each of these drug regimens was associated with decreased in-hospital survival and an increased frequency of ventricular arrhythmias when used for treatment of COVID-19.”
Clinical Take Home Point: This study not only suggests an absence of efficacy for hospitalized patients with COVID-19, but a real signal of harm with increased ventricular arrhythmias with hydroxychloroquine or chloroquine (with or without macrolides) compared to a control population. These medications should simply not be used outside of a randomized clinical trial.
Infographic Created By Mark Ramzy, DO (Twitter: @MRamzyDO)
References:
- Mehra MR et al. Hydroxychloroquine or Chloroquine With or Without a Macrolide for Treatment of COVID-19: A Multinational Registry Analysis. Lancet 2020. [Epub Ahead of Print]
For More on This Topic Checkout:
Post Peer Reviewed By: Anand Swaminathan, MD (Twitter: @EMSwami)
The post COVID-19 Update: Just Say No to Hydroxychloroquine or Chloroquine With or Without Macrolides appeared first on REBEL EM - Emergency Medicine Blog.