
 Background: The COVID-19 Pfizer BNT162b2 vaccine, let’s just call it the COVID-19 Pfizer vaccine (so I don’t have to keep writing BNT162b2) is a novel mRNA vaccine developed to give immunity against the SARS CoV2 virus. It is synthesized mRNA packaged in small lipid nanoparticles but must be stored at extremely low temperatures (-70 C and 2 – 8 C for 5 days ) to prevent degradation. The mRNA encodes for a small portion of the SARS CoV2 virus known as the spike protein and does not encode for the entire virus itself.
Background: The COVID-19 Pfizer BNT162b2 vaccine, let’s just call it the COVID-19 Pfizer vaccine (so I don’t have to keep writing BNT162b2) is a novel mRNA vaccine developed to give immunity against the SARS CoV2 virus. It is synthesized mRNA packaged in small lipid nanoparticles but must be stored at extremely low temperatures (-70 C and 2 – 8 C for 5 days ) to prevent degradation. The mRNA encodes for a small portion of the SARS CoV2 virus known as the spike protein and does not encode for the entire virus itself.
This small lipid nanoparticle is injected into your body and then enters the cell. The lipid nanoparticles serve to protect and preserve the mRNA from degradation and allows it to enter cells readily. After the mRNA enters the cells, the ribosomes will then take the mRNA and begin to synthesize this information to produce the spike protein portion of the SARS CoV-2 virus. The spike protein is what is believed to help the SARS CoV-2 virus enter human cell, replicate, and then lead to the syndrome known as COVID-19. This spike protein is believed to be the immunogenic portion of the virus that the body will then recognize as foreign and begin to develop an immune response against. The vaccine is given in 2 doses that are to be administered 3 weeks apart.
Paper: Polack FP et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. NEJM 2020. PMID: 33301246
Clinical Question: What is the safety and efficacy of BNT162b2 vaccine (The Pfizer COVID-19 Vaccine) against confirmed COVID-19 infection?
What They Did:
- Ongoing, multinational (152 sites worldwide), placebo-controlled, observer blinded, pivotal efficacy trial
- Reporting the safety and efficacy from the Phase 2/3 trial
- Discuss the safety, immunogenicity, and efficacy of the of 30 micrograms of BNT162b2 in preventing COVID-19 in persons >16 years of age
- Randomized 1:1 ratio to receive 2 doses (21 days apart) of either placebo or vaccine
Outcomes:
-
Primary
- Examined the efficacy of the vaccine (BNT162b2) against confirmed COVID-19 with onset of at least 7 days after the second dose in participants who had been without serologic or virologic evidence of SARS-CoV-2 Infection up to 7 days after the second dose
- Confirmed COVID-19: Defined according to FDA criteria as the presence of at least one of the following symptoms: fever, new or increased cough, new or increased shortness of breath, chills, new or increased muscle pain, new loss of taste or smell, sore throat, diarrhea, or vomiting, combined with a respiratory specimen obtained during symptomatic period + positive SARS-CoV-2 testing by nucleic acid amplification
- Adverse events through ≈14 weeks after 2nd dose
- Efficacy in participants with and without evidence of prior infection
-
Secondary:
- Efficacy of the vaccine against severe COVID-19
- Severe COVID-19: Confirmed COVID-19 (see above) + additional features of clinical signs at rest that are indicative of severe systemic illness; Respiratory failure; evidence of shock; significant acute renal, hepatic, or neurologic dysfunction; admission to an intensive care unit; or death.
- Safety & side effects
Inclusion:
- Age ≥16 years
- Healthy or had stable chronic medical conditions
- Examples of chronic stable medical conditions included
- HIV
- Hepatitis B
- Hepatitis C
- Others
Exclusion:
- Medical history of COVID-19
- Treatment of immunosuppressive therapy
- Diagnosis with an immunocompromising condition
Results:
- 44,820 persons were screened
- 43,548 persons >16 years old underwent randomization
- 43,448 participants received injections (21,720 received vaccine vs 21,728 received placebo)
- 152 sites worldwide: US (130 sites), Argentina (1 site), Brazil (2 sites), South Africa (4 sites), Germany (6 sites), and Turkey (9 sites)
- At the data cut-off date of Oct 9th a total of 37,706 participants had a median of at least 2 months of safety data available after the 2nd dose and contributed to the main safey data set
- Safety:
-
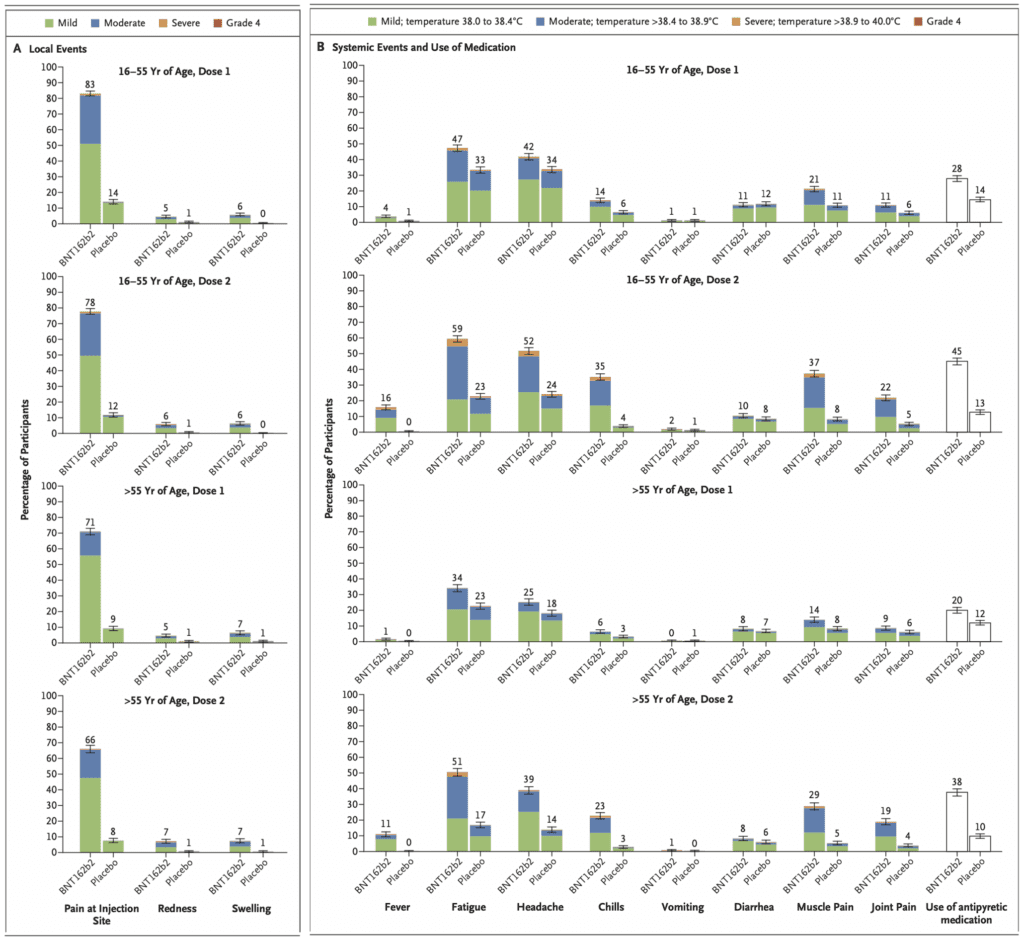
- Adverse effects were divided into local and systemic, ages (16-55 vs >55) as well as doses (Dose 1 vs Dose 2)
-
- Overall, more participants developed local reactions than placebo recipients
- Mild to moderate pain at the injection site within 7d after an injection was the most commonly reported local reaction with <1% of participants across all age groups reporting severe pain
- The proportion of participants reporting local reactions did not increase after the second dose
- No grade 4 local reactions
- Local reactions resolved within 1 to 2 days
- Systemic events were reported more often by younger vaccine recipients (16 to 55 years of age) than older vaccine recipients (>55 years of age)
- Most commonly reported systemic events were fatigue and headache
- Vaccine: 59% & 52% after the 2nd dose among younger recipients
- Vaccine: 51% and 39% after the 2nd dose among older recipients
- Placebo: 23% and 24% after the 2nd dose among younger recipients
- Placebo: 17% and 14% after the 2nd dose among older recipients
- Most commonly reported systemic events were fatigue and headache
- Overall, more participants developed local reactions than placebo recipients
- Safety profile was mostly short-lived mild to moderate pain at the injection site, fatigue and headache
-
- Adverse Events
- More vaccine recipients than placebo recipients reported any adverse event (27% vs 12%) or a related adverse event (21% vs 5%)
- Frequency of any severe systemic event after 1st dose was ≤0.9%
- Incidence of serious adverse events was similar between groups (Vaccine 0.6% vs Placebo 0.5%)
- 64 vaccine recipients (0.3%) vs 6 placebo recipients (<0.1%) reported lymphadenopathy (Generally resolved by 10 days)
- Very few severe adverse events or serious adverse events
- 4 serious adverse events reported in the vaccine group: Shoulder injury from vaccine administration, right axillary lymphadenopathy, paroxysmal ventricular arrythmia, and right leg paresthesia)
- 2 vaccine recipients died (arteriosclerosis, cardiac arrest) but 4 died in the placebo group.
- No deaths were considered related to the vaccine or placebo
- Adverse Events
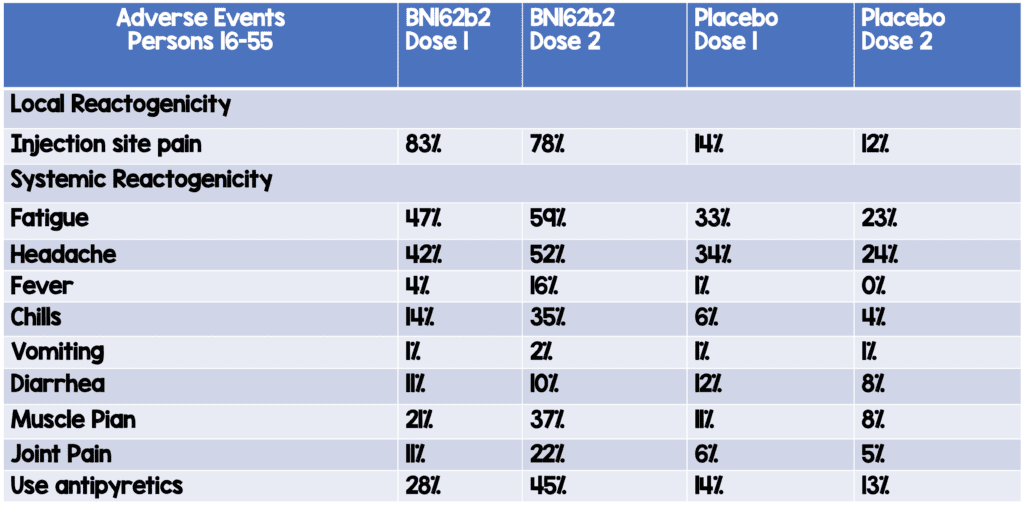
Adverse Events in Participants Aged 15 to 55 Years of Age
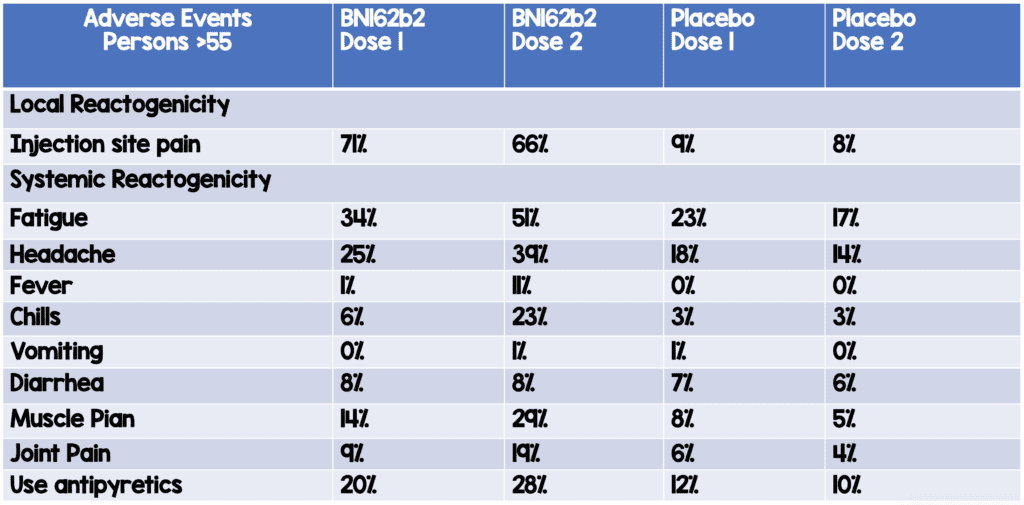
Adverse Events in Participants Aged >55 Years of Age
- Efficacy
-
- Among 36,523 participants who had no evidence of existing or prior SARS-CoV2 infection, 8 cases of COVID-19 with onset at least 7 days after the second dose were observed in the vaccine group and 162 cases were observed in the placebo group giving the vaccine a 95% efficacy (95% CI 90.3 to 97.6)
- The study was not designed to assess efficacy after 1 dose but showed a 52% vaccine efficacy (95% CI 29.5 to 68.4%) indicating early protection by the vaccine starting as soon as 12d after the 1st dose
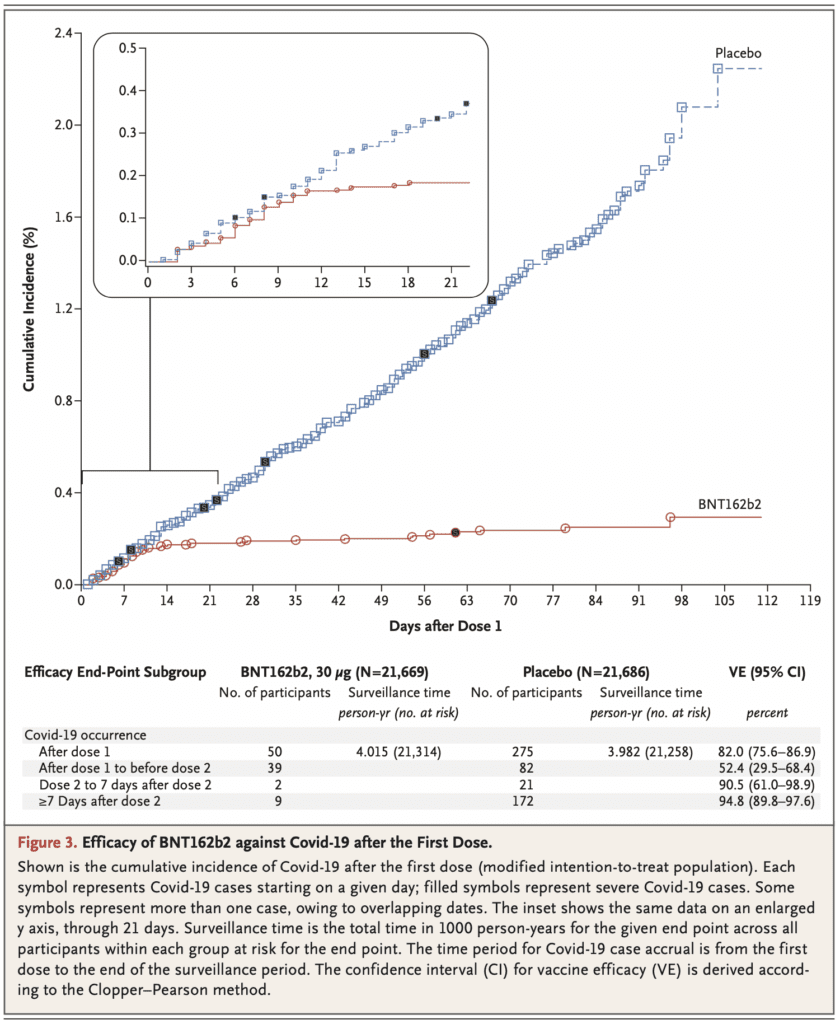
-
- Also not designed to assess efficacy <7 days after the second dose but showed a 91% vaccine efficacy
Strengths:
- Large randomized, multinational, observer-blind, placebo-controlled trial
- Pragmatic trial including patients of all ages and those with chronic stable medical conditions to reflect common recipients of the vaccine
- Good balance of patients between the ages of 16 to 55 (57.8%) and >55 years of age (42.2%)
- Participants with obesity and other coexisting conditions were represented fairly well in this trial
- >40% of participants were >55 years of age, a particularly vulnerable group to COVID-19
Limitations:
- Conducted and funded by Pfizer, the creator of the vaccine, which does not negate the results, but should give some level of pause in interpreting the results
- Longer term effects of the vaccine are not known and there will be both a 2 month follow up on ½ of the trial participants and a 3 ½ month follow up on an even small portion of these recipients
- Study not powered to assess efficacy by subgroups or after a single-dose regimen of vaccination
- The trial does not show prevention in younger patients (16 years of age), pregnant patients, and those who are immunocompromised with the latter being major concerns for health risk for developing COVID-19
- Very few cases of severe COVID-19 observed making it difficult to draw any definitive conclusions about the rare cases that occur in vaccinated participants
- It is unclear from this trial if the vaccine prevents asymptomatic infection, or asymptomatic transmission
- It is also unclear from this trial the duration in which an immune response to immunization will last
- Due to the low number of adverse events in this preliminary publication, the study is not large enough to detect less common adverse events reliably
- Although the vaccine can be stored for up to 5 days at standard refrigerator temperatures once ready for use, very cold temperatures are required for shipping and longer storage. This creates a logistical issue in distribution and storage of the vaccine as every facility may not have the required refrigerators to establish these low temperatures
Discussion:
- The trial demonstrates the approach of using an mRNA vector seems to be a promising approach to vaccine administration
- Although the study was not powered to definitively assess efficacy by subgroups or after a single dose regimen, there was a similar vaccine efficacy (≈90 to 100%) observed across subgroups defined by age, sex, race, ethnicity, baseline BMI, and the presence of coexisting conditions and the cumulative incidence of COVID-19 cases over time begins to diverge by 12 days after the 1st dose of vaccine indicating an early onset of partially protective effect of immunization compared to placebo
- Of the 10 cases of severe COVID-19 that were observed after the 1st dose, only 1 occurred in the vaccine group. This provides preliminary evidence of vaccine-mediated protection against severe disease, which hypothesizes against the theoretical concerns over vaccine-mediated disease enhancement
- Only 1.6% of patients enrolled did not receive 2nd dose of vaccine. Although this number is very low in this trial, it is unclear how well uptake of the 2nd injection will be out in the community that is not optimized like a trial for follow up
- Majority of patients were white (82.9%) in the included study and although not a major limitation, ensuring equity in vaccine distribution will be a key component outside of this clinical trial as it has been reported that higher illness prevalence is seen in minorities
- Although the study was designed to follow participants for safety and efficacy for 2 years after the second dose, the authors state that given the high vaccine efficacy, ethical and practical barriers prevent following placebo recipients for 2 years without offering active immunization. Therefore, long-term safety and efficacy for this vaccine will occur, but it cannot be in the context of maintaining a placebo group
- The paper mentions further data and trials to be reporting efficacy and safety in younger age groups, pregnant, and immunocompromised patients
- Prior to this vaccine, no existing vaccines have been shown to be effective against infection with any betacoronavirus.
Author Conclusion: “A two-dose regimen of BNT162b2 conferred 95% protection against COVID-19 in persons 16 years of age or older. Safety over a median of 2 months was similar to that of other viral vaccines.”
Clinical Take Home Point: The two-dose regimen of BNT162b2 developed by Pfizer seems to be both safe with mostly minor adverse effects and effective against protecting against COVID-19. This trial shows that the production and distribution of vaccine can occur in <12 months’ time. A feat which has never been seen before and demonstrates that both the safety and efficacy of mRNA-based vaccines are a major new tool to combat pandemics and other infectious disease outbreaks.
This is truly a case of the best of times and the worst of times. Although this is an amazing scientific achievement in unprecedented time, it is occurring during a pandemic that is surging out of control and its benefits will most likely not be seen for several months with countless more lives taken.
References:
- Polack FP et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. NEJM 2020. PMID: 33301246
- Rubin EJ et al. SARS-CoV-2 Vaccination – An Ounce (Actually, Much Less) of Prevention. NEJM 2020. [Link is HERE]
For More Info on This Topic Checkout:
-
Pregnancy and the COVID-19 Vaccine Decision Aid:
- Vaccine Info for Pregnant Women (ENGLISH)
- Vaccine Info for Pregnant Women (SPANISH)
- Clinician researchers at the University of Massachusetts medical school (Link is HERE)
- Baystate brought together a working group of experts and pregnant health care workers to rapidly create, design, and disseminate this decision aid
Post Peer Reviewed By: Salim R. Rezaie, MD (Twitter: @srrezaie)
The post COVID-19 Update: The COVID-19 Pfizer Vaccine appeared first on REBEL EM - Emergency Medicine Blog.
