
 Background Information: Over one year into the pandemic many therapies to treat COVID-19 have targeted innumerable aspects of the virus. Most recently, the use of corticosteroids to treat the virus’ excessive inflammatory effects has become the front and center of therapy in patients requiring oxygen therapy.1 The RECOVERY trial showed a mortality benefit when using Dexamethasone in severe cases where oxygen therapy or mechanical ventilation was required.2 Interestingly, compared to other corticosteroids, high doses of Methylprednisolone are actually the preferred agent for anti-inflammation in pulmonary diseases as it achieves a more direct effect on cell membrane associated proteins.3 The authors of the following paper sought to investigate the effectiveness of methylprednisolone compared to Dexamethasone in hypoxemic ICU patients with COVID-19.
Background Information: Over one year into the pandemic many therapies to treat COVID-19 have targeted innumerable aspects of the virus. Most recently, the use of corticosteroids to treat the virus’ excessive inflammatory effects has become the front and center of therapy in patients requiring oxygen therapy.1 The RECOVERY trial showed a mortality benefit when using Dexamethasone in severe cases where oxygen therapy or mechanical ventilation was required.2 Interestingly, compared to other corticosteroids, high doses of Methylprednisolone are actually the preferred agent for anti-inflammation in pulmonary diseases as it achieves a more direct effect on cell membrane associated proteins.3 The authors of the following paper sought to investigate the effectiveness of methylprednisolone compared to Dexamethasone in hypoxemic ICU patients with COVID-19.
Paper: Ko JJ, et al. A Comparison of Methylprednisolone and Dexamethasone in Intensive Care Patients With COVID-19. J Intensive Care Med. 2021 Feb 25. PMID: 33632000
Clinical Question:
- Can sufficiently dosed methylprednisolone provide further mortality benefit in ICU patients with COVID-19 when compared to dexamethasone?
What They Did:
- Single center retrospective analysis performed at a large public hospital from March 1st 2020 to July 31st 2020 in California
- Initially proposed a course of high dose, short-term methylprednisolone to treat COVID-19 ICU patients with severe hypoxemia and the plan was to compare this to “usual care” (ie. patients not receiving steroids). Due to the surge of COVID-19 cases and overwhelming workload resulting in IRB approval delays, they transitioned their proposal to a retrospective observational analysis.
- Following results of the RECOVERY trial, the study proposal was then further modified to include the following three groups:
- Group 1: Methylprednisolone = 1 mg/kg/day for > 3 days
- Group 2: Dexamethasone = 6 mg/day for > 7 days
- Group 3: Usual care (no steroid treatment)
- Usual care at the study institution was defined as the following:
- Anticoagulation based on D-Dimer levels
- Prone positioning when feasible for both non-intubated and mechanically ventilated patients when P/F ratio <150 (16hrs prone alternating with 8hrs supine)
- 5 Day course of antibiotics for pneumonia if procalcitonin was >0.25 ng/mL
- Some patients were started on Remdesivir or convalescent plasma
- Very few patients received hydroxychloroquine or tocilizumab
- None of the study patients received ECMO
Inclusion Criteria:
- All COVID-19 positive patients ≥18 years of age and admitted to the ICU for respiratory failure from COVID-19
- Criteria for ICU admission was based on severity of hypoxemia which the authors defined as any of the following:
- An oxygen requirement > 40 L/min and FiO2 > 50%
- Need for mechanical ventilation
- Worst P/F ratio
- Highest positive end expiratory pressure in cmH2O required (determined to be 5 cm H20 for patients on HFNC)
Exclusion Criteria:
- Patients under the age of 18 years old
- Incidental COVID-19 positive patients admitted to the ICU for non-COVID19 related complaints such as:
- Trauma and other surgical conditions
- Diabetic ketoacidosis (DKA) requiring insulin drips
- Drug/alcohol intoxication
- Cardiac events
- Non-COVID19 related sepsis
- GI bleeds requiring endoscopy
Outcomes:
Primary
- All-cause mortality within 50 days of initial treatment
Secondary
- ICU length of stay
- 28-day mortality
Results:

- Of the 285 ICU admissions, a total of 262 patients were included in the final analysis
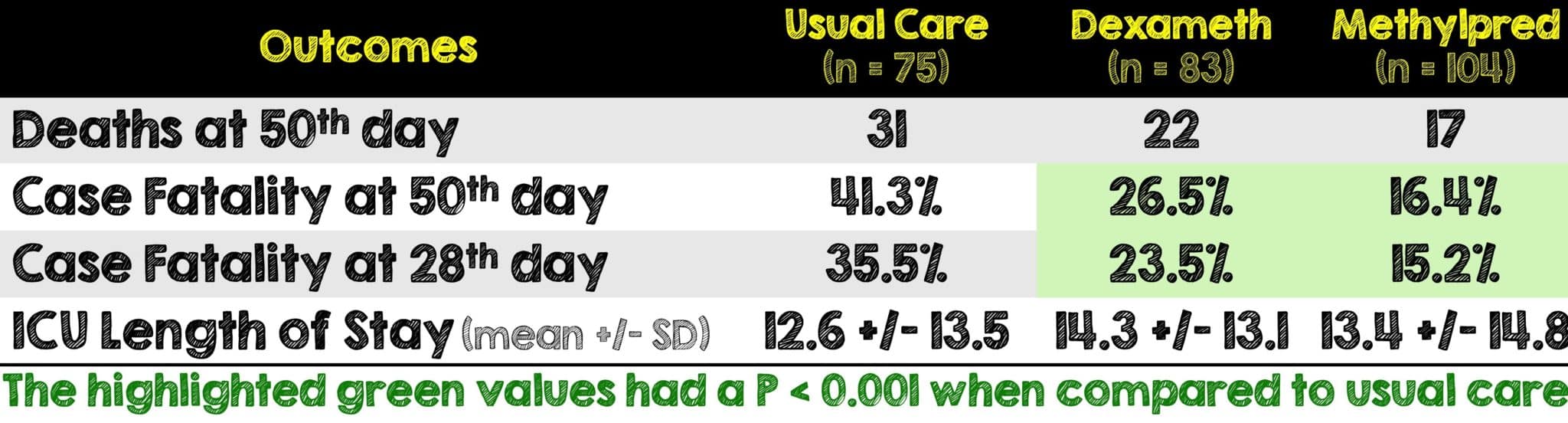
Critical Results:

Strengths:
- Clinically relevant and important question to answer
- Included several objective criteria to define hypoxemia
- Utilized already existing evidence on the benefits of steroids in COVID-19 patients
- Modified study design following the results of the RECOVERY trial
- Patient-oriented primary outcome of all-cause mortality within 50-days of initial treatment
- Had 4-levels of clinical categories to determine a patient’s need for anticoagulation
- Accounted for and analyzed the same patients who were started on Remdesivir or convalescent plasma
- Severity of hypoxemia was balanced and comparable across all three groups
- Potential confounding variables were similarly distributed across all the groups and thus limited their impact on their final conclusion
Limitations:
- Single center study in a single country thus limiting external validity
- Retrospective observational analysis which cannot determine causation
- Small sample size
- Overestimation of severity in usual care group (intubated patients early in pandemic)
- Demographic imbalance to all the patients as majority were Hispanic males
- Did not report any possible side-effects or complications that may have been related to steroid use
Discussion:
- The authors answer a very important question related to which steroid to use in COVID-19 patients, especially given the fluctuating intensity of the current pandemic
- After the RECOVERY trial showed a mortality benefit using Dexamethasone, this study’s design was modified to include methylprednisolone. The authors added methylprednisolone because they saw a strong therapeutic benefit compared to dexamethasone in their preliminary results. It’s unclear and not mentioned anywhere whether these results were included in this study and they are not located in the supplemental material
- In patients requiring mechanical ventilation, methylprednisolone had a superior mortality benefit to dexamethasone and made no statistical difference in patients not requiring mechanical ventilation. This is clinically important if a patient not on mechanical ventilation is having worsening hypoxemia as it may be worthwhile to make a switch from dexamethasone to methylprednisolone as it appears to be associated with a lower mortality.
- Important to remember is that this is a retrospective analysis. Additional verification in prospective, multicenter trials are required and ideally with a much larger sample size
- The severity of patients in the usual care group was very likely overestimated since we were intubating everyone at the start of the pandemic for fear of aerosolization. The authors try to soften this limitation by stating that their study period extended till the end of July however the transition of management to HFNC was delayed well into the end of the spring for the same fear of aerosolization.
- The authors did well in listing several criteria to define hypoxemia. End-organ perfusion, specifically mental status was omitted. Given the unreliability of pulse oximetry in COVID-19 patients, altered mental status and confusion arguably have become very important determinants to aggressive care and prognosis
- There was demographic imbalance in the patient population (ie. majority being hispanic males) and potential confounding variables (ie. older age, presence of comorbidities and requirement of mechanical ventilation). This along with questionable effective COVID-19 therapies (ie. Remdesivir and Convalescent plasma) was accounted for and found to have no impact on the final conclusion
- The findings of this study reinforced the results of the RECOVERY trial by showing that either steroid can be used to reduce mortality among COVID-19 patients who did not require mechanical ventilation
- The results of the RECOVERY trial have placed a high demand on Dexamethasone, leading to nationwide shortages. This study’s findings have the potential to alleviate that demand by offering methylprednisolone as an alternative therapy to decrease mortality in mechanically ventilated COVID-19 patients
- Lastly, an added benefit when using Methylprednisolone over Dexamethasone is the short course of treatment. This has the potential to limit systemic side effects (hyperglycemia, leukocytosis, etc) typically seen with longer durations of corticosteroid use.
Author’s Conclusions:
- In COVID-19 patients requiring mechanical ventilation, sufficiently dosed methylprednisolone can lead to a further decreased mortality as compared to dexamethasone.
Our Conclusion:
- Although a retrospective analysis at a single center with a small sample size, the findings of this study show an association with a decrease in mortality with sufficiently dosed Methylprednisolone compared to Dexamethasone in mechanically ventilated COVID-19 patients admitted to the ICU with severe hypoxemia. Furthermore, the shorter treatment duration of Methylprednisolone may minimize systemic side effects typically seen with corticosteroid use. Both steroids were shown to decrease mortality with no significant difference between the two in non-intubated COVID-19 patients also admitted to the ICU for severe hypoxemia.
Clinical Bottom Line:
- In this single center retrospective analysis, Methylprednisolone was associated with a decreased mortality benefit when compared to Dexamethasone when used in mechanically ventilated COVID-19 patients admitted to the ICU for severe hypoxemia. Methylprednisolone should be considered as the initial corticosteroid of choice in mechanically ventilated patients or patients with acutely worsening hypoxemia who are not mechanically ventilated as it is a shorter course of treatment with the potential to limit systemic side effects.
REFERENCES:
- Ko JJ, et al. A Comparison of Methylprednisolone and Dexamethasone in Intensive Care Patients With COVID-19. J Intensive Care Med. 2021 Feb 25. PMID: 33632000
- Horby P, et al. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021 Feb 25. PMID: 32678530
- Frey FJ, et al. Altered plasma protein-binding of prednisolone in patients with nephrotic syndrome. Am J Kidney Dis. 1984 Mar; PMID: 6702820
For More Thoughts on This Topic Checkout:
- REBEL EM: Dexamethasone in COVID-19: The RECOVERY Trial
- First10EM: Steroids in COVID-19
- PulmCrit: Timed & titrated use of steroid in COVID-19?
Post Peer Reviewed By: Salim Rezaie, MD (Twitter: @Srrezaie)
The post Dexamethasone vs Methylprednisolone in ICU Patients with COVID19 appeared first on REBEL EM - Emergency Medicine Blog.
