
 Background: Venous thromboembolism (VTE) occurs frequently in patient with cancer. Treatment in this group entails a number of challenges including a higher rate of thrombosis recurrence and a higher risk of bleeding. Standard therapy at this time for both symptomatic and asymptomatic VTE is with low-molecular-weight heparin (LMWH) based on results from the CLOT trial (Lee 2003). In non-cancer patients, new oral anticoagulants (NOACs) like rivaroxaban have been shown to be effective in treatment without increasing bleeding events. The NOACs also add ease of use for the patient. Though these agents are frequently used in the treatment of cancer-associated VTE, there is a dearth of evidence supporting this practice.
Background: Venous thromboembolism (VTE) occurs frequently in patient with cancer. Treatment in this group entails a number of challenges including a higher rate of thrombosis recurrence and a higher risk of bleeding. Standard therapy at this time for both symptomatic and asymptomatic VTE is with low-molecular-weight heparin (LMWH) based on results from the CLOT trial (Lee 2003). In non-cancer patients, new oral anticoagulants (NOACs) like rivaroxaban have been shown to be effective in treatment without increasing bleeding events. The NOACs also add ease of use for the patient. Though these agents are frequently used in the treatment of cancer-associated VTE, there is a dearth of evidence supporting this practice.
Article: Raskob GE et al. Edoxaban for the Treatment of Cancer-Associated Venous Thromboembolism. NEJM 2018 PMID: 29231094
Clinical Question: Is edoxaban non-inferior to LMWH in the treatment of cancer-associated VTE?
Population: Adult patients with active cancer or cancer diagnosed within the previous 2 years with acute symptomatic or asymptomatic deep-vein thrombosis (DVT) or pulmonary embolism (PE).
Outcomes:
- Primary: Composite of recurrent VTE (DVT or segmental or more proximal PE) or major bleeding (overt bleeding associated with 2g/dL drop in hemoglobin or a transfusion of two or more units of blood during 12-month follow up.
- Secondary: Clinically relevant non-major bleeding (CRNB), event-free survival, VTE-related death, all-cause mortality, recurrent DVT, recurrent PE.
Intervention: LMWH X 5 days followed by oral edoxaban 60 mg Q24
Control: SQ dalteparin 200 IU/kg Q24 X 1 month followed by 150 IU/kg Q24
Design: Open-label, randomized, non-inferiority trial
Excluded: Need for thrombectomy or insertion of a caval filter, treated with anticoagulation for another indication, active bleeding or other contraindication to anticoagulation, creatinine clearance < 30 ml/min, history of heparin induced thrombocytopenia, acute hepatitis, chronic hepatitis, liver cirrhosis, life expectancy < 3 months, platelet count < 50,000, uncontrolled hypertension, women of childbearing age without proper contraception, chronic NSAID treatment, aspirin therapy (> 100 mg/day) or dual antiplatelet therapy, use of a P-gp inhibitor
Primary Results:
-
1050 patients randomized
- 1046 patients included in modified intention-to-treat analysis
- Edoxaban group: n = 522
- Daleparin group: n = 524
- Baseline characteristics well matched
Critical Findings:
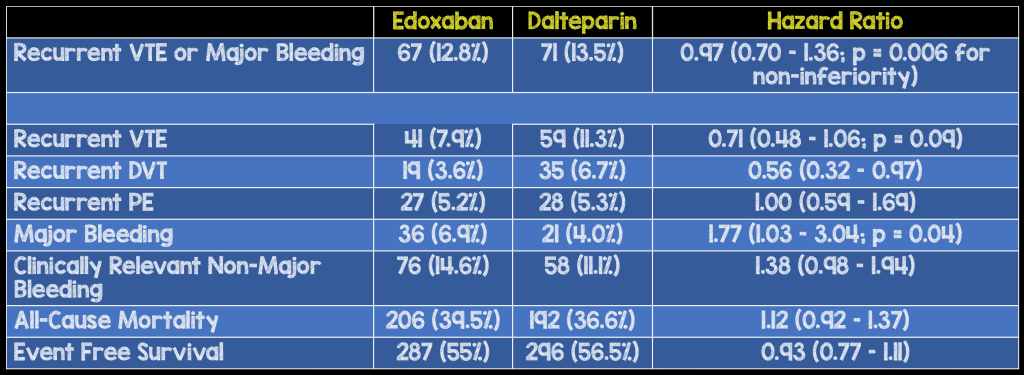
Strengths:
- Asks a clinically relevant, patient centered question. Though not explicitly investigated, patients would likely prefer an oral medication to self-administered SQ injections (in this study, patients decided to quit study drug due to inconvenience of dosing in 4% of edoxaban group vs 14.9% in the dalteparin group)
- Non-inferiority a reasonable goal given comparison of a new approach to standard practice
- Randomization technique was appropriate
- Intention-to-treat analysis for primary outcome better approximates real-life circumstances
- Independent clinical events committee, whose members were unaware of the treatment assignments, confirmed the qualifying diagnosis of VTE
Limitations:
- Study was funded by the manufacturer of edoxaban and the manufacturer worked in coordination with the coordinating committee on trial design, protocol and oversight. This introduces a significant opportunity for influencing the trial outcomes
- Composite primary endpoint combining an efficacy outcome (recurrent VTE) with an adverse event rate (major bleeding). Additionally, the adverse outcome is of far more clinical importance than the efficacy outcome (unbalanced outcomes in composite)
- A search on clinicaltrials.gov revealed that the study originally listed recurrent VTE and major bleeding as co-primary endpoints not as a composite endpoint. The primary outcome was altered after planning of the study. Additionally, the original study design listed recurrent VTE at 6 months (not 12 months as written in the study) as the primary endpoint.
- Study was non-blinded which may introduce significant bias from both clinicians and patients. For instance, the possibility of recurrent VTE may have been evaluated differently based on what medication the patient was taking
- Death from any cause was higher in the edoxaban group but not statistically significantly higher. A larger study may have shown that the ~ 3% difference in mortality favoring dalteparin is real
Discussion:
- Breakdown of the primary outcome demonstrated a lower recurrent VTE rate in the edoxaban group (higher efficacy) with a higher major bleed rate (higher adverse event rate) but a non-statistically significant difference in event-free survival
- Even if we believe that bias inherent in industry funded, open-label trials is insignificant, a lower recurrent VTE rate isn’t as important as an increased major bleed rate. Thus, it’s hard to argue non-inferiority
- The original primary outcome was altered after planning of the trial. This may have been done after researchers recognized that the study would not show non-inferiority in it’s original design. Recurrent VTE as an outcome was altered from 6 month follow up to 12 month follow up. Again, this alteration may have been undertaken after the researchers realized that at 6 months, there was no difference between dalteparin and edoxaban (as evidenced by figure 2)
- The study conclusions appear purposely written to stress the benefit and decrease stress on the harms.
- The difference in the combination of death or major bleeding was not reported. Based on the reported outcomes, that number would sit at 5.8%
- The median duration of treatment was shorter with dalteparin than with edoxaban, which may have improved efficacy outcomes and increased bleeding rates in the edoxaban group vs dalteparin
- Major bleeding seems to be driven by upper GIB and mostly in patients who entered the trial with GI cancer
Authors Conclusions:
“Oral edoxaban was noninferior to subcutaneous dalteparin with respect to the composite outcome of recurrent venous thromboembolism or major bleeding. The rate of recurrent venous thromboembolism was lower but the rate of major bleeding was higher with edoxaban than with dalteparin.”
Our Conclusions: Edoxaban treatment resulted in a higher major bleed rate in patients with cancer associated VTE along with a lower recurrent VTE rate.
Potential to Impact Current Practice: This study should not be used to change practice as it shows increased significant harms (major bleeding, death) with only a small benefit in terms of recurrent VTE.
Bottom Line: LMWH should remain the standard treatment over edoxaban in cancer-associated VTE. This topic is an important one for continued research as NOACs clearly are preferable from an ease of use perspective. Future studies should have more thoughtful (and honest) considerations for the primary endpoint.
For More on this Topic Checkout:
- Pulm CCM: DVT-PE in Cancer: Oral Anticoagulant Edoxaban Non-Inferior to Enxaparin
- The SGEM: SGEM #235 – Edoxaban for Cancer Associated VTE – Would the NEJM Lie to You?
References:
- Lee AY et al. Low-molecular-weight heparin versus coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. NEJM 2003; 349(2): 146-53. PMID: 12853587
Post Peer Reviewed By: Salim R. Rezaie, MD (Twitter: @srrezaie)
The post Edoxaban in Cancer-Associated VTE appeared first on REBEL EM - Emergency Medicine Blog.
