 The Coronaviridae family and its genera coronaviruses have been implicated as having neurotropic and neuroinvasive capabilities in human hosts (Bohmwald 2018). They have been associated with the development of neuropsychiatric symptoms, seizure activity, encephalomyelitis, acute flaccid paralysis, cerebral venous sinus thrombosis, Guillain-Barré syndrome, as well as cerebrovascular disease (Bohmwald 2018, St Jean 2004).
The Coronaviridae family and its genera coronaviruses have been implicated as having neurotropic and neuroinvasive capabilities in human hosts (Bohmwald 2018). They have been associated with the development of neuropsychiatric symptoms, seizure activity, encephalomyelitis, acute flaccid paralysis, cerebral venous sinus thrombosis, Guillain-Barré syndrome, as well as cerebrovascular disease (Bohmwald 2018, St Jean 2004).
Recently, there has been a growing body of evidence supporting the association of SARS-CoV2 with neurological abnormalities. A systematic review looking at the incidence of secondary neurological disease in patients diagnosed with SARS-CoV2 found rates to vary from 6-36.4% (Herman 2020).
At the time of this submission, there have been ten reports of acute transverse myelitis (ATM) attributed to SARS-CoV2, and others are currently being submitted or are in pre-print at this time (See infographic below).
ATM has a varied presentation and is associated with significant morbidity and mortality that necessitates increased awareness and vigilance on the part of the clinician. This has become especially important in light of a possible causal link of ATM to SARS-CoV2 with emerging cases during the COVID-19 pandemic.
Here, we review the salient features of infectious ATM (both para-infectious and post-infectious) to increase recognition of this disease entity.

Definition: Transverse myelitis is defined as an inflammatory myelopathy that is associated with a variety of different etiologies, both compressive and non-compressive.
Infectious acute transverse myelitis is a subtype that is defined as an acute inflammation of the spinal cord precipitated by an infectious insult (including SARS-CoV2) resulting in neurological deficits.
 Epidemiology:
Epidemiology:
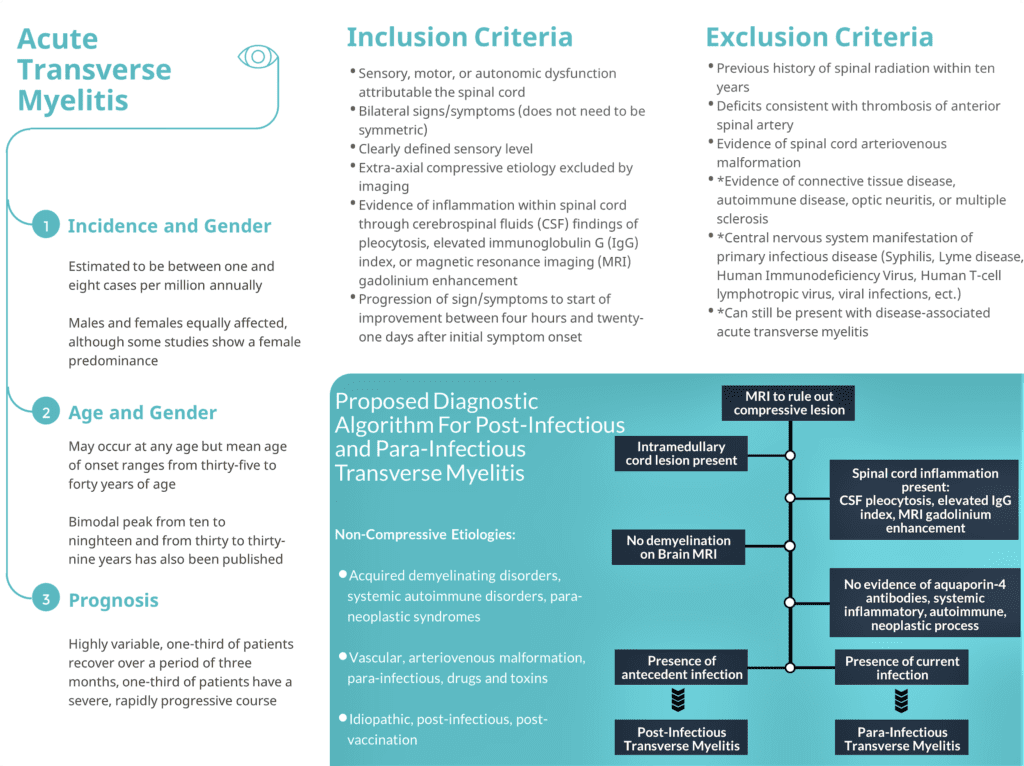
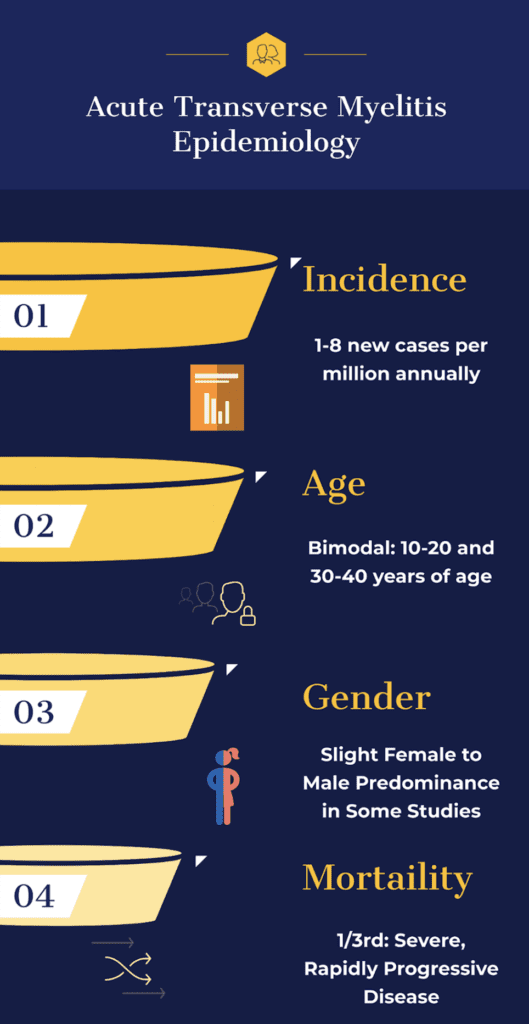
Incidence: Overall incidence rate of 1-8 new cases per million annually but recent data shows that it may be as high as 31 cases per million (Borchers 2012, Frohman 2010)
- 20-30% of cases of ATM occur in the pediatric population
- Retrospective multi-center study estimated rate of infectious or para-infectious ATM to be 17.3% overall (de Seze 2005)
Age: Bimodal peak at 10 to 20 years of age and at 30 to 40 years of age with mean age of onset between 35 and 40 years of age (Borchers 2012)
Gender: Most studies show that men and women are equally affected with some studies showing a female patient predominance (Borchers 2012, Frohman 2010)
Morbidity and Mortality:
- Highly variable with one third of patients exhibiting a severe and rapidly progressive disease course
- One third of patients experience a good outcome with complete recovery
- The remaining one third of patients experience mild to moderate functional and ambulatory deficits (Borchers 2012)
Poor Prognostic Factors:
- Back pain
- Time to maximal deficit within 24 hours
- Longitudinal spinal cord involvement (Borchers 2012)
Pathogenesis:
The current pathogenesis of ATM secondary to SARS-CoV2 is unknown. Some proposed hypotheses are listed below.
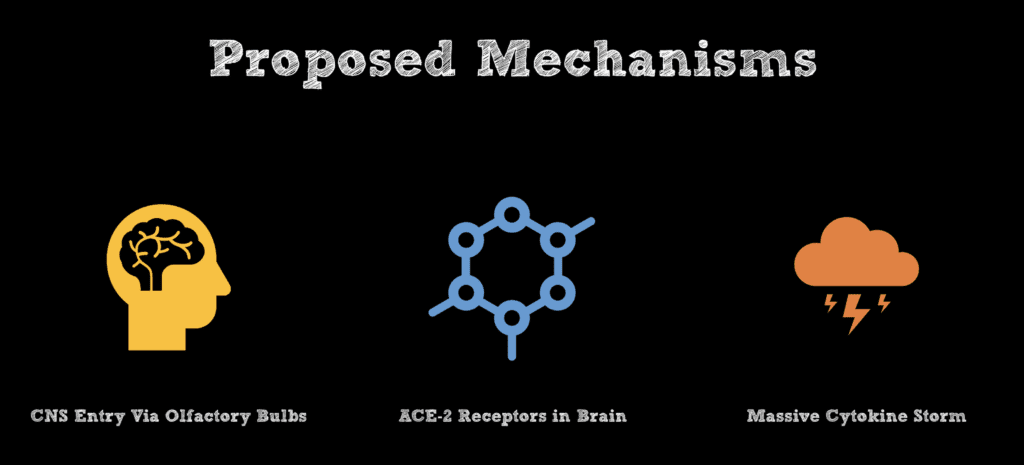
- Proposed Hypothesis: Previous studies in mice have proposed that human coronavirus may reach the CNS via the olfactory bulbs followed by propagation and detection in whole brain tissue days later through retrograde axonal spread. It is hypothesized that subsequent viral infection of CNS glial and neuronal cells triggers demyelination as well as an inflammatory response (Bohmwald 2018).
- Proposed Hypothesis: Interaction of SARS-CoV2 with angiotensin-converting enzyme 2 (ACE2) expressed within glial cells and spinal neurons (Bang 2020).
- Proposed Hypothesis: Indirect damage to the central nervous system through massive cytokine release causing downstream cerebral autoregulation disruption (Jenny 2019).
History and Physical:
Risk Factors:
- Post-infectious or para-infectious ATM requires exposure to an infectious source
- Patients may or may not exhibit symptoms of their inciting infection
- SARS-CoV2 ATM: exposure, symptoms, or diagnosis of SARS-CoV2 increases risk
Symptoms: ATM may have a varied motor, sensory, and autonomic neurological symptoms and is easily missed.
- Symptoms may evolve over hours to days
- The thoracic spinal cord is most commonly affected location (Frohman 2010)
- The most common symptoms include autonomic bladder symptoms (almost 100%), lower limb paresthesia (80-95%), allodynia (80%), paraparesis (50%), and back pain (30-50%) (Awad 2011)
-
Motor weakness: typically, bilateral and below a well-defined spinal cord level
- Unilateral syndromes have also been described
- Classically, upper motor neuron signs such are hyperreflexia and presence of Babinski sign are noted.
- A rapid-onset, severe ascending paraparesis or quadriparesis with areflexia and hypotonia may occur in the acute phase making diagnosis difficult
-
Sensory abnormalities: well defined truncal sensory level below which sensation of pain and temperature is lost.
- Ascending paresthesia have also been noted up to the level of the spinal cord lesion
-
Autonomic dysfunction:
- Bowel and bladder dysfunction
- Orthostatic hypotension
- Temperature dysregulation
- Sexual dysfunction
Diagnosis:
Diagnosis of post-infectious or para-infectious ATM is based upon uniform diagnostic criteria published by the Transverse Myelitis Consortium Working Group (TMCWG 2002).
These criteria rely on the exclusion of extra-axial compressive etiology along with inclusion of bilateral sensory, motor, or autonomic dysfunction attributed to a clearly defined spinal cord sensory level with evidence of spinal cord inflammation through either cerebrospinal fluid (CSF) or magnetic resonance imaging (MRI) findings (TMCWG 2002).
Inclusion Criteria:
- Development of sensory, motor, or autonomic dysfunction attributable to the spinal cord
- Bilateral signs and/or symptoms (though not necessarily symmetric)
- Clearly defined sensory level
- Exclusion of extra-axial compressive etiology by neuroimaging (MRI or myelography; CT of spine not adequate)
- Inflammation within the spinal cord demonstrated by CSF pleocytosis or elevated IgG index or gadolinium enhancement. If none of the inflammatory criteria is met at symptom onset, repeat MRI and lumbar puncture evaluation between 2- and 7-days following symptom onset meet criteria
- Progression to nadir between 4 h and 21 day following the onset of symptoms (if patient awakens with symptoms, symptoms must become more pronounced from point of awakening)
Exclusion Criteria:
- History of previous radiation to the spine within the last 10 years
- Clear arterial distribution clinical deficit consistent with thrombosis of the anterior spinal artery
- Abnormal flow voids on the surface of the spinal cord consistent with AVM
- Serologic or clinical evidence of connective tissue disease or autoimmune disease (does not exclude disease-associated transverse myelitis)
- Brain MRI abnormalities suggestive of multiple sclerosis (does not exclude disease-associated transverse myelitis)
- History of clinically apparent optic neuritis (does not exclude disease-associated transverse myelitis)
Labs – routine labs are unhelpful in the emergency department diagnosis but are drawn to assist inpatient management and rule out other etiologies
- Serum: CBC, CMP, UA with culture, Blood cultures, HIV, RPR, TSH, ESR/CRP, serological targeted testing for infectious causes (Frohman 2010)
- Autoimmune Workup: NMO-IgG antibodies, ANA, RF, ANCA (Beh 2013)
- Lumbar Puncture:
- Full workup includes CSF cell count with differential, protein, glucose, VDRL, IgG index, oligoclonal bands, cytologic analysis, culture (Beh 2013)
- The presence of pleocytosis or an elevated IgG index is consistent with an inflammatory myelitis (Frohman 2010)
- Viral myelitis will often show a predominately lymphocytic pleocytosis and elevated CSF protein level (Kincaid 2006)
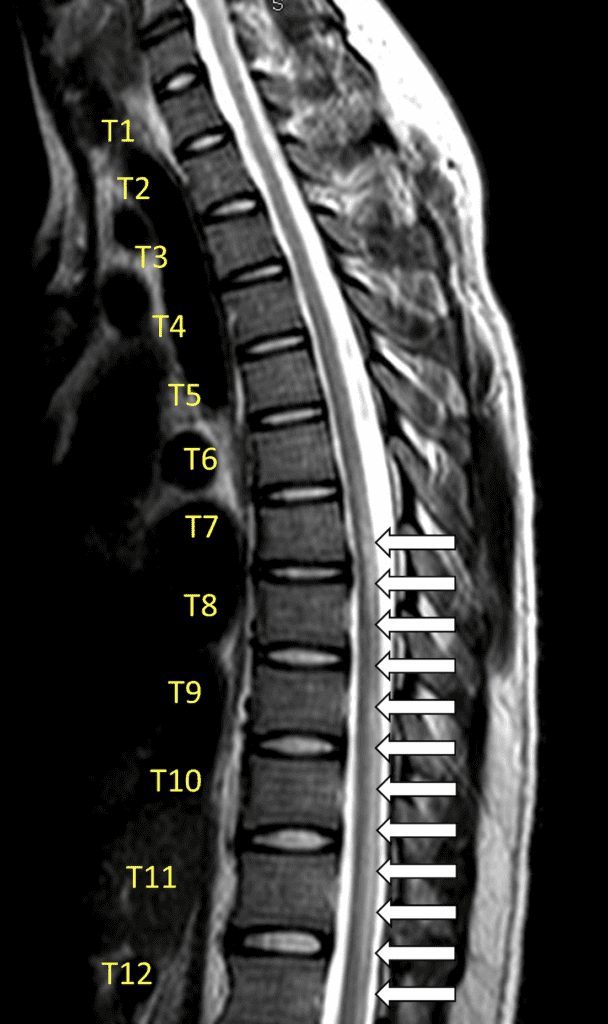
Imaging
- Chest radiography: to evaluate for presence of infectious pulmonary etiology
- Gadolinium enhanced MRI of the entire spine: Gold standard for diagnosis (Frohman 2010)
- Serves to rule out compressive or structural etiologies needing surgical decompression
- Once extra-axial compressive etiologies are excluded, MRI may show evidence of spinal cord inflammation characterized by an intramedullary spinal cord lesion with gadolinium enhancement
- Classically, MRI demonstrates T2-hyperintense spinal cord lesions extending over more than two segments and involving more than two-thirds the cross-sectional area of the cord (Goh 2011)
- MRI of the brain with and without gadolinium: If suspicion for multiple sclerosis or suspicion of demyelination extending beyond the spinal cord is present (Borchers 2012)
- CT myelography: limited ability to visualize spinal cord makes this imaging modality useful only if MRI is contraindicated (Borchers 2012).
Management: Goal of treatment during the acute phase is to address the underlying para-infectious cause if present and to halt the progression of the inflammatory spinal cord lesion (Frohman 2010)
- Corticosteroids: Standard first-line treatment, yet no randomized, controlled trials have been done and supportive evidence comes from case studies and expert opinion (Frohman 2010).
- High dose intravenous methylprednisolone 1000 mg daily for 3-7 days
- Evidence of a specific corticosteroid or route of administration is currently lacking (Frohman 2010, Borchers 2012)
- Oral regimens have been used in patients with mild episodes of myelitis (Frohman 2010)
- Plasma Exchange: Rescue therapy in patients without response to corticosteroids (Frohman 2010)
- Use of plasma exchange is supported by randomized trials in severe cases that are unresponsive to pulse corticosteroid use (Awad 2011)
- Cyclophosphamide and IVIG
- Currently there is no evidence to support use of cyclophosphamide for myelitis and supporting evidence is derived from retrospective, underpowered studies (Awad 2011)
- Similarly, there is no high-quality evidence to support routine use of IVIG
Take Home Points:
Guest Post By:

Muhammad Durrani, DO, MS
Assistant Clerkship Director, Assistant Research Director
Inspira Medical Center Emergency Department
Vineland, NJ
Twitter: @IbbyDurrani
References:
- Bohmwald K et al. Neurologic alterations due to respiratory virus infections. Front Cell Neurosci 2018. PMID: 30416428
- St-Jean J et al. Human respiratory coronavirus OC43: genetic stability and neuroinvasion. J Virol 2004. PMID: 15280490
- Herman C et al. Scoping review of prevalence of neurologic comorbidities in patients hospitalized for COVID-19. Neurology 2020. PMID: 32345728
- Alkebti R et al. Acute myelitis as a neurological complication of Covid-19: A case report and MRI findings. Radiol Case Rep 2020. PMID: 32685076
- Chow C et al. Acute transverse myelitis in COVID-19 infection. BMJ Case Rep 2020. PMID: 32784242
- Durrani M et al. Acute Transverse Myelitis Secondary to Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): A Case Report. Clin Pract Cases Emerg Med 2020. Link Here
- Munz M et al. Acute Transverse Myelitis After COVID-19 Pneumonia. J Neurol 2020. PMID: 32458198
- Paterson R et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain 2020. PMID: 32637987
- Sarma D et al. A case report of acute transverse myelitis following novel coronavirus infection. Clin Pract Cases Emerg Med 2020. Link Here
- Valiuddin H et al. Acute transverse myelitis associated with SARS-CoV-2: A Case-Report. Brain Behav Immun Health 2020. Link Here
- Zachariadis A et al. Transverse myelitis related to COVID-19 infection. J Neurol 2010. PMID: 32601756
- Zhao K et al. Acute myelitis after SARS-CoV-2 infection: a case report. medRxiv 2020. Link Here
- Borchers A et al. Transverse myelitis. Autoimmun Rev 2012. PMID: 21621005
- Frohman E et al. Clinical practice. Transverse myelitis. N Engl J Med 2010. PMID: 20818891
- de Seze J et al. Idiopathic acute transverse myelitis: application of the recent diagnostic criteria. Neurology 2005. PMID: 16380618
- Bang A et al. Evidence of the COVID-19 Virus Targeting the CNS: Tissue Distribution, Host-Virus Interaction, and Proposed Neurotropic Mechanisms. ACS Chem Neurosci 2020. PMID: 32167747
- Jenny N et al. Inflammatory cytokines and ischemic stroke risk: The REGARDS cohort. Neurology 2019. PMID: 31004072
- Awad A et al. Idiopathic transverse myelitis and neuromyelitis optica: clinical profiles, pathophysiology and therapeutic choices. Curr Neuropharmacol 2011. PMID: 22379456
- Transverse Myelitis Consortium Working Group (TMCWG). Proposed diagnostic criteria and nosology of acute transverse myelitis. Neurology 2002. PMID: 12236201
- Beh S et al. Transverse myelitis. Neurol Clin 2013. PMID: 23186897
- Kincaid O et al. Viral myelitis: an update. Curr Neurol Neurosci Rep 2006. PMID: 17074281
- Goh C et al. Neuroimaging in acute transverse myelitis. Neuroimaging Clin N Am 2011. PMID: 22032509
Post Peer Reviewed By: Anand Swaminathan, MD (Twitter: @EMSwami) and Salim R. Rezaie, MD (Twitter: @srrezaie)
The post Infectious Acute Transverse Myelitis Secondary to COVID-19 appeared first on REBEL EM - Emergency Medicine Blog.