
 Background: Venous thromboembolism (VTE) occurs frequently in patient with cancer. Treatment in this group entails a number of challenges including a higher rate of thrombosis recurrence and a higher risk of bleeding. Standard therapy in 2018 for both symptomatic and asymptomatic VTE is with low-molecular-weight heparin (LMWH) based on this study. Prior to 2003, patients were treated with warfarin after bridging with either unfractionated or LMWH. This approach requires frequent monitoring due to unpredictable anticoagulation levels associated with drug interactions, malnutrition and vomiting. Due to these issues, treatment with LMWH alone may be both more efficacious as well as preferred by patients.
Background: Venous thromboembolism (VTE) occurs frequently in patient with cancer. Treatment in this group entails a number of challenges including a higher rate of thrombosis recurrence and a higher risk of bleeding. Standard therapy in 2018 for both symptomatic and asymptomatic VTE is with low-molecular-weight heparin (LMWH) based on this study. Prior to 2003, patients were treated with warfarin after bridging with either unfractionated or LMWH. This approach requires frequent monitoring due to unpredictable anticoagulation levels associated with drug interactions, malnutrition and vomiting. Due to these issues, treatment with LMWH alone may be both more efficacious as well as preferred by patients.
Article: Lee AYY et al. Low-molecular-weight heparin versus coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. NEJM 2003; 349(2): 146-53. PMID: 12853587
Clinical Question: What is the rate of recurrent VTE in patients with cancer who are treated with dalteparin (LMWH) in comparison to an oral vitamin K antagonist (warfarin or acenocoumarol)?
Population: Adult patients with active cancer (diagnosis within 6 months, treatment within the last 6 months or recurrent or metastatic cancer) and newly diagnosed VTE (symptomatic proximal (calf veins excluded) DVT or PE or both)
Outcomes:
- Primary: Objectively documented, symptomatic recurrent VTE within 6 months
- Secondary: Clinically overt bleeding (both major bleeding and any bleeding)
Intervention: Dalteparin 200 IU/kg (max of 18,000 IU) Q24 X 1 month then with 75-83% of full dose X 5 months
Control: Dalteparin 200 IU/kg (max of 18,000 IU) Q24 along with warfarin or acenocoumarol (vitamin K antagonist). Dalteparin was discontinued after a minimum of 5 days and once INR > 2.0 for two consecutive days
Design: Randomized, open-label study
Excluded: Patients with basal or squamous-cell carcinoma, weight < 40 kg, ECOG performance score of 3 or 4, already received heparin for more than 48 hours, already on oral anticoagulation, serious bleeding within the last 2 weeks, platelet count < 75,000, contraindication to heparin, contraindication to contrast medium, creatinine level > three times upper limit, pregnancy or could not return for follow up.
Primary Results:
-
1303 patients initially met inclusion criteria
- 439 patients met one or more exclusion criteria
- 864 patients ultimately eligible and 676 provided written consent
-
676 patients randomized
- Dalteparin: n = 338
- Oral Anticoagulation: n = 338
- 2 patients from each arm excluded after randomization for not having a qualifying VTE
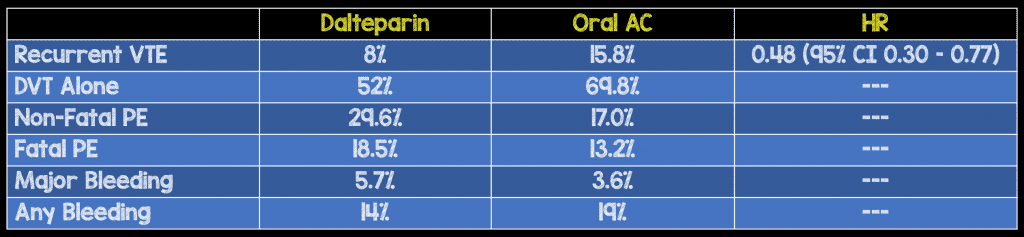
Critical Findings:
Strengths:
- Asks a clinically important, patient centered question
- Multicenter, multinational study which increases external validity
- Robust follow up with telephone calls every two weeks plus seen in clinic at one week, one, three, and six months after randomization
- Baseline characteristics were similar between groups
- Recurrent VTE had to be objectively documented
- All potential events (recurrent VTE + bleeding) were reviewed by central adjudication committee who were blinded to treatment assignment
Limitations:
- Randomization process was not detailed in the manuscript
- Unclear if patients were enrolled consecutively or not
- Providers and patients were not blinded to treatment arm which may introduce biases
- The pharmaceutical company funded the study but it’s unclear from the manuscript how involved they were in design or decision process
Discussion:
- 20/53 VTE in the oral anticoagulant group occurred when the patient’s INR was < 2.0 demonstrating both the importance of adequate anticoagulation as well as the difficulty in staying in the therapeutic range
- Initially, it may seems that taking an oral anticoagulant would be preferable to injections but, compliance was no worse in LMWH group. This likely reflects inconvenience of frequent testing, dietary changes etc with vitamin K antagonists
Authors Conclusions:“In patients with cancer and acute venous thromboembolism, dalteparin was more effective than an oral anticoagulant in reducing the risk of recurrent thromboembolism without increasing the risk of bleeding.”
Our Conclusions: We agree with the authors conclusions. Dalteparin appears to be at least as good and likely better than oral vitamin K antagonists for the prevention of recurrent VTE in patients with active cancer. Clearer methodology and blinding would make this study more powerful.
Potential to Impact Current Practice: In this case, the impact is already clear. The CLOT study led to a paradigm shift in the treatment of cancer-associated VTE. Standard practice for the last decade has been treatment with LMWH though this is now shifting with the advent of NOACs.
Clinical Bottom Line: LMWHs like dalteparin should be considered first line treatment in cancer-associated VTE.
References:
- Lee AYY et al. Low-molecular-weight heparin versus coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. NEJM 2003; 349(2): 146-53. PMID: 12853587
Post Peer Reviewed By: Salim R. Rezaie, MD (Twitter: @srrezaie)
The post LMWH in Cancer-Related VTE (CLOT Study) appeared first on REBEL EM - Emergency Medicine Blog.
