 Background: While the effects of stroke can be both debilitating and devastating, the benefit of certain treatments have not always been clear and the data supporting them are fraught with controversy. Reanalysis of the two trials demonstrating the benefit of thrombolytics in the treatment of stroke (NINDS-21 and ECASS III2) produced results that challenge the originally stated benefits of this treatment approach.3,4 For further reading on this topic, you can refer to the blog post by Salim Rezaie on REBEL EM here.
Background: While the effects of stroke can be both debilitating and devastating, the benefit of certain treatments have not always been clear and the data supporting them are fraught with controversy. Reanalysis of the two trials demonstrating the benefit of thrombolytics in the treatment of stroke (NINDS-21 and ECASS III2) produced results that challenge the originally stated benefits of this treatment approach.3,4 For further reading on this topic, you can refer to the blog post by Salim Rezaie on REBEL EM here.
Endovascular treatment is the other means of acute intervention used to treat large vessel occlusion acute stroke. The initial benefits of this treatment within 6 hours of the onset of symptoms were demonstrated in the MR CLEAN trial.5 In this trial, patients managed with intraarterial treatment had a higher rate of functional independence (defined as a modified Rankin Score of 0 to 2), 32.6%, when compared to standard of care (thrombolytics in 89% of cases), 19.1%. These results translate into a 13.5% absolute increase in functional independence in the intraarterial treatment group. While this was a well done study, it is not without its limitations which are discussed in further detail in blogs by Ryan Radecki, St. Emlyn’s, and Rory Spiegel. Unfortunately, the results of this trial led to the other trials evaluating the benefits of endovascular therapy (EXTEND-IA6 and ESCAPE7) being stopped early due to ethical concerns. While both trials demonstrated a benefit in endovascular therapy, the risk of stopping a trial early is that it may demonstrate a larger difference between two groups than one that truly exists.
Further trials were conducted using advanced imaging techniques to identify patients that may benefit from endovascular therapy between 6 and 24 hours of last known well. The DAWN8 trial demonstrated a benefit in functional independence defined using a utility-weighted modified Rankin score at 90 days in 49% of patients receiving treatment compared to 13% in the control group. In the DEFUSE-39 trail, the time from last known well was 6-16 hours. The patients who received medical therapy in addition thrombectomy had improved functional neurological outcomes (modified Rankin Score of 0 to 2) when compared to medical therapy alone, 45% vs 17%, respectively. Both trials were unfortunately terminated early. Based on these data, it does appear that there is a true benefit to endovascular therapy in the select patient population presenting with a large vessel occlusion acute stroke based on advanced imaging demonstrating a lesion amenable to this therapy. Currently, the American Heart Association/American Stroke Association guidelines recommend mechanical thrombectomy for the situations based on the results of these trials.10
With the demonstrated benefit of endovascular therapy in select patient populations and the questionable efficacy of thrombolysis, the question that remains is if there is a benefit to treatment with alteplase in patients with who qualify for endovascular therapy?
Paper: A Randomized Trial of Intravenous Alteplase before Endovascular Treatment for Stroke (MR CLEAN-NO IV)11
Clinical Question: Is EVT alone more effective, or noninferior, as compared to intravenous alteplase followed by EVT in European patients with acute ischemic stroke due to an intracranial anterior-circulation stroke who present to an EVT-capable center?
What They Did:
- 20 hospitals in the Netherlands, Belgium, and France
- Investigator-initiated, prospective, open-label, multicenter, randomized trial in Europe in patients with stroke who presented directly to a hospital capable of providing endovascular therapy (EVT) who qualified for both EVT and alteplase based on current treatment guidelines.
- 1:1 randomization of EVT alone or intravenous alteplase followed by EVT (standard of care)
Outcomes:
- Primary: Functional outcome on the modified Rankin scale at 90 days ( 14 days) after randomization, analyzed for superiority and then for noninferiority
- Secondary:
- Recanalization on the first intracranial angiogram
- Successful reperfusion on the final intracranial angiogram
- Recanalization on CTA or MRA at 24 hours
- NIHSS score at 24 hours and at 5 to 7 days or discharge (if earlier)
- Final lesion volume on MRI at 24 hours or noncontrast CT at 5 or 7 days or at discharge
- A comparison within three dichotomized groups with respect to the score on the modified Rankin scale (0 or 1 vs. 2 to 6, 0 to 2 vs. 3 to 6, 0 to 3 vs. 4 to 6) at 90 days
- Score on the EuroQol Group 5-demension 5-Level Self-Report questionnaire at 90 days
- Score on the Barthel Index at 90 days
- Safety End Points:
- Any intracranial hemorrhage and symptomatic cerebral hemorrhage according to the Heidelberg Bleeding Classification
- Postprocedure femoral artery aneurysm or groin hematoma
- Embolization to new cerebral territory
- Infarction in new cerebral vascular territory on noncontrast CT at 5 to 7 days or discharge or on diffusion-weighted MRI at 24 hours
- Death from any cause within 90 days
Inclusion Criteria:
- 18 years of age or older
- NIHSS ≥2
- Admitted directly to a center that performed EVT
- Eligible for EVT and IV alteplase within 4.5 hours after symptom onset according to local guidelines
- Acute ischemic stroke due to an intracranial proximal occlusion of the anterior circulation as assessed by CTA or MRI
- Intracranial internal carotid artery
- First segment of the middle cerebral artery (M1)
- Proximal second segment of the middle cerebral artery (M2)
Exclusion Criteria:12
- BP > 185/110 mmHg
- Blood glucose < 2.7 mmol/L (48.6 g/dL) or > 22.2 mmol/L (399.6 g/dL)
- Cerebral infarction in the previous 6 weeks with residual neurologic deficit or signs of recent infarction on neuroimaging
- Recent head trauma; recent major surgery or serious trauma
- Use of vitamin K antagonist with an INR > 1.7
- Known thrombocytopenia with platelets < 100 x 109/L
- Treatment with direct thrombin or factor X inhibitors
- Treatment with a therapeutic dose of (low-molecular weight) heparin
- Prestroke disability that interferes with the assessment of functional modified Rankin score at 90 days
- Participation in another clinical trial with the exception of the MR ASAP Trial (multicenter randomized trial of acute stroke treatment with a nitroglycerin patch)
Methods:
- 1:1 randomized based on permuted blocks stratified according to center and whether they were included in the MR ASAP Trial (9 patients included in both trials)
- Usual-care group
- 9 mg/kg body weight (maximum 90 mg), 10% as a bolus and the remaining 90% over an hour
- EVT (could be initiated before the alteplase infusion was completed)
- EVT-alone
- Could receive 0.9 mg/kg alteplase as a rescue medication if there was incomplete reperfusion after EVT, within 4.5 hours of symptom onset
- Primary end-point data was collected by telephone interviews performed by trained research nurses unaware of the trial-group assignments
Statistics:
- Sample size calculation:
- Distribution of modified Rankin scale (mRS) based on the results of the original MR CLEAN trial.5
- Assumed treatment effect with a common odds ratio (cOR) of 1.54 (the absolute risk difference of a mRS of 0-2 of approximately 8%)
- Non-inferiority calculation:
- Lower boundary of a 95% confidence interval of the cOR of 0.8
- 5% certainty that EVT alone does not differ more than 5% in the percent of patients with a mRS of 0 to 2 in favor of standard treatment
- Translation: if there are fewer than 5% of patients in the EVT alone group with a mRS of 0 to 2 compared to standard treatment, it is non-inferior
- Superiority calculation based on a modified intention-to-treat population
- Primary effect calculation:
- cOR, with a 95% confidence interval, for a shift in the direction of better outcomes on the mRS (estimated with ordinal logistic regression).
- Adjusted for age, baseline NIHSS, collateral status, prestroke score mRS, time from symptom onset to randomization
- Lower boundary of a 95% confidence interval of the cOR of 0.8
Results:
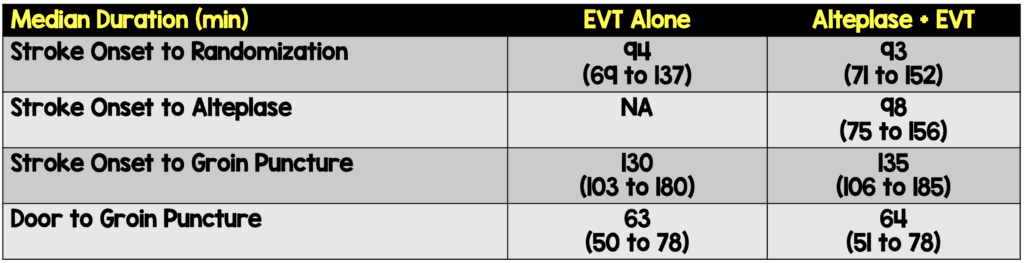
- Patients:
- 547 patients randomized, 539 with deferred consent were included in the modified intention-to-treat analysis.
- Data missing for 7 patients.
- 26 (4.8%) did not get EVT (EVT alone, n=12 vs. alteplase + EVT, n=14)
- EVT alone: 273
- Slightly higher age
- Higher percentage of atrial fibrillation
- Higher percentage of terminal internal carotid occlusions
- 7% received alteplase before EVT (n=10)
- 3% received rescue IV alteplase (n=19)
- Alteplase followed by EVT: 266
- 7% did not receive alteplase before EVT (n=10)
- Median age: 71
- 6% male
- EVT alone: 273
- Primary End Point
- EVT-alone: median mRS of 3 (2-5)
- Alteplase + EVT: median mRS of 2 (2-5)
- Adjusted odds ratio for a shift in mRS = 0.84 (0.62 to 1.15; P=0.28)
- EVT alone is not superior to alteplase followed by EVT
- Because the low end of the 95% CI is lower than 0.8 (0.62), EVT alone is not non-inferior to alteplase followed by EVT
- Secondary end points
- Successful reprfusion
- EVT Alone: 78.7%
- Alteplase Followed by EVT: 83.1%
- aOR 0.73; 95% CI 0.47 to 1.13
- Safety (EVT-alone vs alteplase + EVT)
- Mortality at 90 days: 20.5% vs 15.8% (adjusted odds ratio, 1.39; 0.84 to 2.3)
- Symptomatic intracranial hemorrhage: 5.9% vs. 5.3% (adjusted odds ratio, 1.30; 0.60 to 2.81)
- Any intracranial hemorrhage: ~35% in both groups.
- Successful reprfusion
Strengths:
- Asks a clinically relevant question
- Addresses the external validity of previous trials in China and Japan demonstrating no difference between EVT and alteplase + EVT
- Research nurses performing mRS assessments were blinded to treatment groups
- Statistics performed by an independent statistician
- Sources providing funding for the study were not involved in the design, planning, analysis, or reporting of the data
- Assumption that omitting intravenous alteplase before EVT would reduce the risk of intracerebral hemorrhage
Limitations:
- Open label trial design (would have been possible to blind the alteplase infusion and unblind if needed for rescue treatment)
- No screening logs, so it is unclear how many patients need to be screened before they meet criteria for this treatment
- Only included patients presenting to a EVT capable hospital – lacks external validity to hospitals not capable of providing this treatment
- EVT only group was higher risk (older, more a-fib, worse occlusion location)
- Power to calculate noninferiority (99%) is higher than the power to calculate superiority (91%)
- Low interrater reliability of mRS
- Unrestricted industry funding
Discussion:
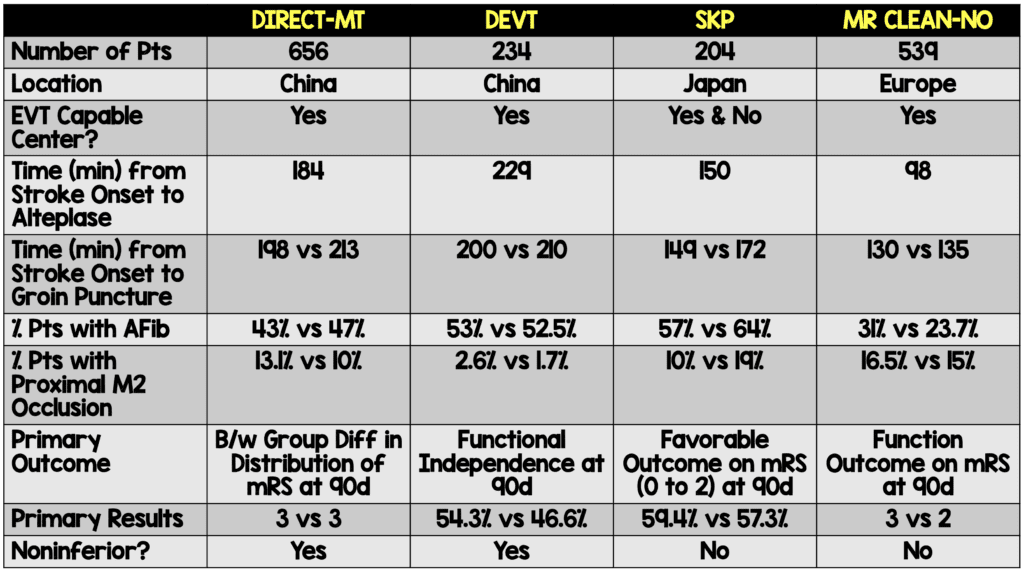
When interpreting these results, we must evaluate where it fits into what we already know about the subject. There have been three trials to date evaluating the question of EVT vs alteplase followed by EVT. Two studies conducted in China, DIRECT-MT and DEVT, demonstrated that alteplase followed by EVT was noninferior to EVT alone.13,14 The SKIP Trial, performed in Japan using a smaller dose of alteplase (0.6 mg/kg), demonstrated similar favorable functional outcomes in both groups (59.4% in the EVT alone group and 57.3% in the alteplase followed by EVT group), but fell short of proving noninferiority. As the authors of this study state, the wide confidence interval around the results also did not show inferiority.15
The patient population difference between these studies and the current one is that the previous trials were conducted in China and Japan in which the patient population may not be generalizable to a European (or North American) population, as was conducted in this study. Patients enrolled in the SKIP trial were not always at a center capable of performing EVT and some patients received alteplase prior to transport. All the patients enrolled in the MR CLEAN-NO IV trial started at a center capable of providing endovascular therapy. Because of this, the results from this trial do not address the question of the benefit (or noninferiority) of providing alteplase to patients presenting with a large vessel occlusion to a facility not capable of providing endovascular therapy. Further discussion on the DEVT and SKIP trials can be found on a post by Dr. Swaminathan on REBEL EM.
Other differences between the previous trials and the current one are:
- The time to alteplase in the MR CLEAN-NO IV trial was shorter than in the previously discussed trials.
- The number of patients with a history of atrial fibrillation was lower in this trial than previous trials.
- If the EVT alone did not result in successful reperfusion, patients were allowed to receive rescue alteplase (n=19).
- Patients randomized in the EVT only group had higher risk factors for worse outcome including being older, having more rates of atrial fibrillation, and a higher risk occlusion location.
The big question regarding the results of this trial is, what does it mean to be not superior but also not noninferior? The first part is straightforward. Based on the prespecified endpoint of a common odds ratio of 1.54 (approximately 8% difference in mRS of 0 to 2 between groups), EVT alone, is not superior to alteplase followed by EVT. The difference between groups was 0.84 (0.62 to 1.15; P=0.28). The odds ratio includes 1, meaning that there is not a statistically significant difference between the two groups. In fact, the secondary efficacy end points demonstrate that there was an absolute difference 2% in mRS of 0 to 2 between groups (49.1% vs 51.1% in the EVT and alteplase followed by EVT group, respectively; OR 0.95; 95% CI 0.65 to 1.39). From this we can conclude, based on these results, there is no statistically difference between groups and EVT alone is not superior to alteplase followed by EVT.
Unfortunately, the statistical analysis of the results don’t stop here. Instead, this is combined with a noninferiority evaluation of the same results. When conducting a noninferiority trial, there must be some inherit benefit in the treatment being evaluated. In this case, there is a potential benefit to not using alteplase prior to EVT. Alteplase is an expensive medication, it is associated with an increased risk of intracranial hemorrhage, and it has questionable efficacy based on the reanalysis of NINDS-2 as well as ECASS III.3,4 Based on these potential benefits, it does appear to be an appropriate use of a noninferiority analysis.
To perform the analysis adequately, the margin of noninferiority must be calculated a priori (as was performed in this study), the study must be powered to determine noninferiority a priori (not done in this study, it was evaluated after the superiority analysis), and must be clinically relevant. The 0.8 margin appears to be a similar margin for determining noninferiority when compared to other trials. Although the results were an aOR 0.84 (above the prespecified noninferiority margin), because the 95% CI was from 0.62 to 1.15, there is a chance that the true value is less than 0.8 (EVT is inferior to alteplase followed by EVT), meaning the results are not noninferior. Because the confidence interval is also greater than 1, there is also a chance that EVT is superior to alteplase followed by EVT. The confidence interval also includes 1 meaning there is no statistical difference between the two groups. The interpretation of noninferiority analysis of these data are that EVT is not noninferior to alteplase followed by EVT, but neither is it superior, nor is it inferior.
It must also be noted that in the pre-published protocol, noninferiority was a secondary outcome, meaning it is only hypothesis generating.12 Not only that, these results are based on the adjusted common odds ratio using ordinal logistic regression for age, baseline NIHSS, collateral status, prestrike score on the mRS, and time from symptom onset to randomization. All these together increase the risk that a difference between the groups has been found outside of the noninferiority margin that may not be clinically significant.
Author Conclusion:
“In a randomized trial involving European patients, EVT alone was neither superior nor inferior to intravenous alteplase followed by EVT with regard to disability outcome at 90 days after stroke. The incidence of symptomatic intracerebral hemorrhage was similar in the two groups.”
Conclusion:
In European patients presenting to a comprehensive stroke center capable of performing EVT, EVT alone is not superior nor is it inferior to treatment with alteplase followed by EVT. There was no statistical difference between these two groups. Further research is needed before we can definitively conclude EVT alone is noninferior to alteplase followed by EVT at an EVT capable center, but at this point, we have 4 clinical trials demonstrating that it is not inferior to perform EVT without alteplase (This study does not address treatment with alteplase prior to transport to an EVT capable center).
Guest Post By:

Wilcox Medical Center
Kaua`i, HI
References:
- National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue Plasminogen Activator for Acute Ischemic Stroke. NEJM. 1995. PMID: 7477192.
- Hacke W, et al. Thrombolysis with Alteplase 3 to 4.5 Hours After Acute Ischemic Stroke. NEJM. 2008. PMID: 18815396.
- Hoffman JR, Schriger DL. A Graphic Reanalysis of the NINDS Trial. Ann Emerg Med. 2009. PMID: 19464756.
- Alper BS, et al. Thrombolysis with Alteplase 3-4.5 Hours After Acute Ischaemic Stroke: Trial Reanalysis Adjusted for Baseline Imbalances. BMJ Evid Based Med. 2020. PMID: 32430395; PMCID: PMC7548536.
- Berkhemer OA, et al. A Randomized Trial of Intraarterial Treatment for Acute Ischemic Stroke. NEJM. 2015. PMID: 25517348.
- Campbell BC, et al. Endovascular Therapy for Ischemic Stroke with Perfusion-Imaging Selection. NEJM. 2015. PMID: 25671797.
- Goyal M, et al. Randomized Assessment of Rapid Endovascular Treatment of Ischemic Stroke. NEJM. 2015. PMID: 25671798.
- Nogueira RG, et al. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. NEJM. 2018. PMID: 29129157.
- Albers GW, et al. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. NEJM. 2018. PMID: 29364767; PMCID: PMC6590673.
- Powers WJ, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019. PMID: 31662037.
- LeCouffe NE, et al. A Randomized Trial of Intravenous Alteplase before Endovascular Treatment for Stroke. NEJM. 2021. PMID: 34758251.
- Treurniet KM, et al. MR CLEAN-NO IV: intravenous Treatment Followed by Endovascular Treatment Versus Direct Endovascular Treatment for Acute Ischemic Stroke Caused by a Proximal Intracranial Occlusion-Study Protocol for a Randomized Clinical Trial. Trials. 2021. PMID: 33588908; PMCID: PMC7885482.
- Yang P, et al. Endovascular Thrombectomy with or without Intravenous Alteplase in Acute Stroke. NEJM. 2020. PMID: 32374959.
- Zi W, et al. Effect of Endovascular Treatment Alone vs Intravenous Alteplase Plus Endovascular Treatment on Functional Independence in Patients With Acute Ischemic Stroke: The DEVT Randomized Clinical Trial. JAMA. 2021. PMID: 33464335; PMCID: PMC7816099.
- Suzuki K, et al. Effect of Mechanical Thrombectomy Without vs With Intravenous Thrombolysis on Functional Outcome Among Patients With Acute Ischemic Stroke: The SKIP Randomized Clinical Trial. JAMA. 2021. PMID: 33464334; PMCID: PMC7816103.
Post Peer Reviewed By: Salim R. Rezaie, MD (Twitter: @srrezaie)
The post MR CLEAN-NO IV: Endovascular Treatment for Stroke Compared to Alteplase Followed by Endovascular Treatment: No Difference, But Also Not Not Worse appeared first on REBEL EM - Emergency Medicine Blog.