 Background: Acute gastrointestinal bleeding (GIB) is a common diagnosis dealt with by emergency clinicians. Definitive therapy for acute GIB often includes endoscopy or surgery. However, there is a myriad of pharmaceutical options (i.e. PPI, Somatostatin Analogues, Antibiotics, etc.) as well as blood products that may be instituted as part of the acute resuscitation of these patients. The role of tranexamic acid (TXA) in resuscitation of this condition is unknown.
Background: Acute gastrointestinal bleeding (GIB) is a common diagnosis dealt with by emergency clinicians. Definitive therapy for acute GIB often includes endoscopy or surgery. However, there is a myriad of pharmaceutical options (i.e. PPI, Somatostatin Analogues, Antibiotics, etc.) as well as blood products that may be instituted as part of the acute resuscitation of these patients. The role of tranexamic acid (TXA) in resuscitation of this condition is unknown.
TXA has become one of the darling medications of emergency medicine, with numerous indications, minimal side effect profile and low cost. TXA works by inhibiting blood clot breakdown (i.e. fibrinolysis). TXA has been shown to decrease death from bleeding in other conditions (Trauma, Postpartum hemorrhage) but there is limited evidence for its use in GIB. A systematic review and meta-analysis of seven randomized trials with just over 1600 patients [2] showed a reduction in all-cause mortality. However, the individual trials were small and prone to a myriad of biases making these conclusions hypothesis generating at best.
REBEL Cast Episode 85 – The HALT-IT Trial – TXA in Acute GI Bleeds
Click here for Direct Download of Podcast
Paper: The HALT-IT Trial Collaborators. Effects of High-Dose 24-h Infusion of Tranexamic Acid on Death and Thromboembolic Events in Patients with Acute Gastrointestinal Bleeding (HALT-IT): An International Randomised, Double-Blind, Placebo-Controlled Trial. Lancet 2020. [Epub Ahead of Print]
Clinical Question: Does IV tranexamic acid reduce 5-day death due to bleeding in adult patients with acute gastrointestinal hemorrhage compared to placebo?
What They Did:
- International, multicenter, randomized, placebo-controlled trial in 164 hospitals in 15 countries
- Patients randomly assigned to:
- TXA: Loading dose of 1g of TXA in 100mL infusion of 0.9% sodium chloride over 10 min, followed by a maintenance dose of 3g TXA added to 1L of isotonic IV solution infused at 125mg/hr for 24hrs
- Placebo: Matching 0.9% sodium chloride
Outcomes:
-
Primary: Death due to bleeding within 5 days of randomization
- Analysis excluded patients who received neither dose of the allocated treatment and those for whom data on death were unavailable
-
Secondary:
- Death due to bleeding within 24h and 28d of randomization
- All-cause and cause specific mortality at 28d
- Rebleeding within 24h, within 5d, and within 28d of randomization, surgery or radiological intervention
- Blood product transfusion
- Arterial Thromboembolic Events (MI or Stroke)
- Venous Thromboembolic Events (DVT or PE)
- Seizures
- Other complications (including cardiac events, sepsis, pneumonia, respiratory failure, renal failure, liver failure)
- Days in the ICU
- Functional status in-hospital or at 28d
Inclusion:
- Clinician uncertain whether to use TXA
- Older than minimum age to be considered an adult (Either ≥16 years or ≥18 years)
- Significant upper and/or lower GIB (Defined as risk of bleeding to death)
- Hypotension
- Tachycardia
- Signs of shock
- Likely to need transfusion, urgent endoscopy, or surgery
Exclusion:
- Not stated
Results:
- 12,009 patients randomized
- TXA: 5994 patients
- Placebo: 6015 patients
- 11,952 (99.5%) received the 1st dose of the allocated treatment
- 89% of patients had upper GIB
- 55% of patients had suspected variceal bleeding
- 9% of patients were on anticoagulation
- 41% of patients had liver disease
-
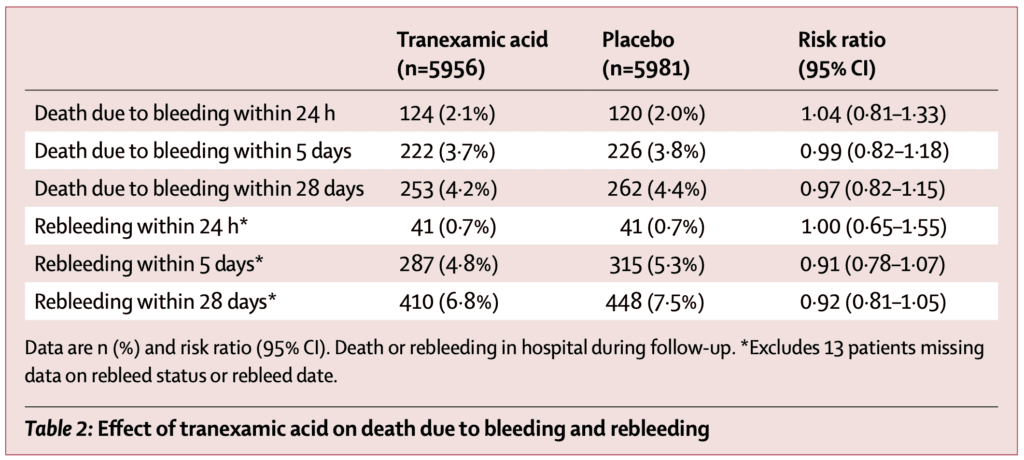
5d Mortality due to bleeding (Primary Outcome):
- TXA: 222 patients (4.0%)
- Placebo: 226 patients (4.0%)
- RR 0.99; 95% CI 0.82 to 1.18)
- No difference in any of the subgroups of the primary outcome
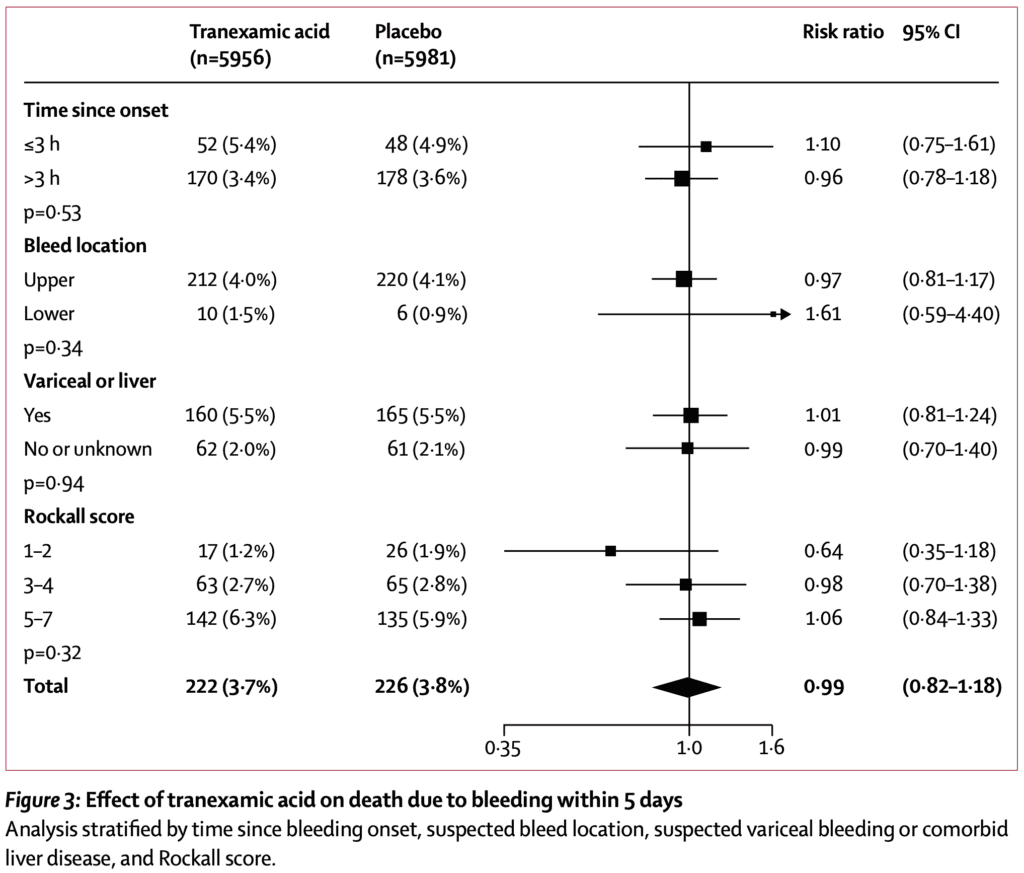
- No difference in death due to bleeding or rebleeding at different time points
- Arterial Thromboembolic Events (MI or Stroke):
- TXA: 42 patients (0.7%)
- Placebo: 46 patients (0.8%)
- RR 0.92; 95% CI 0.60 to 1.39
- Venous Thromboembolic Events (DVT or PE):
- TXA: 48 patients (0.8%)
- Placebo: 26 patients (0.4%)
- RR 1.85; 95% CI 1.15 to 2.98
- NNH = 250
- Proportion of patients who had surgery, radiological intervention and blood product transfusion similar in both groups:
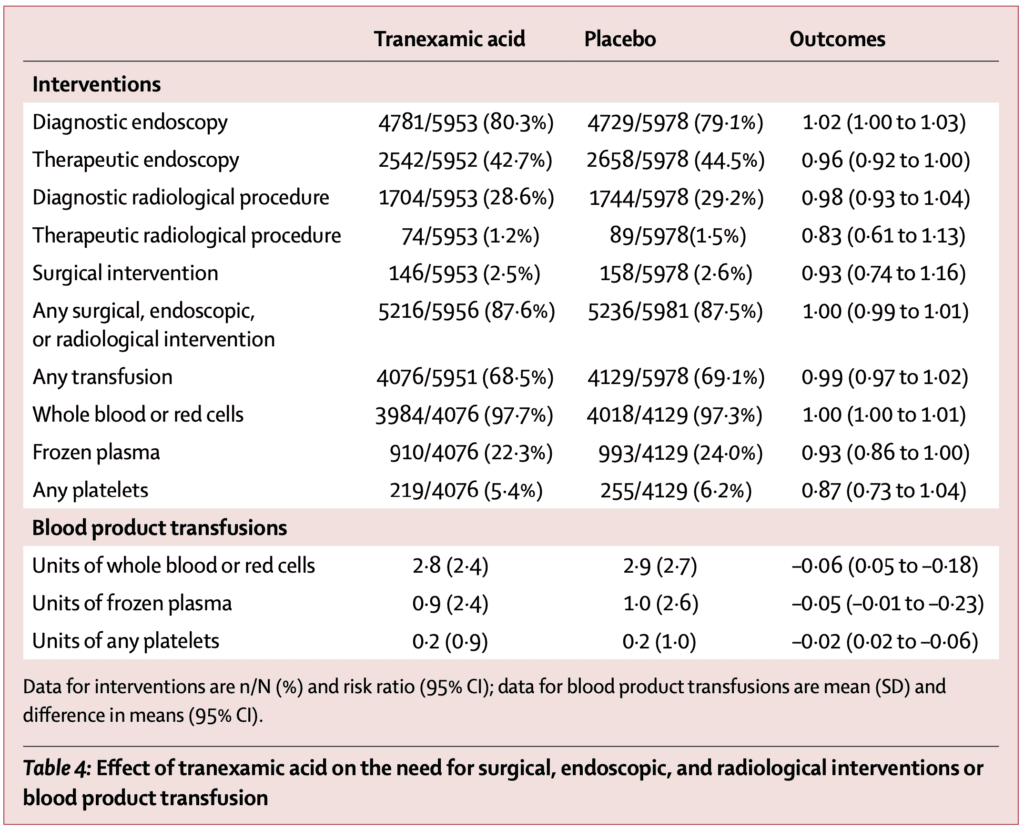
- Seizures
- TXA: 38 patients (0.6%)
- Placebo: 22 patients (0.4%)
- RR 1.73; 95% CI 1.03 to 2.93
- Also excluded patients not receiving maintenance dose and found 33 events vs 17 events (RR 1.95; 95% CI 1.09 to 3.50)
Strengths:
- Largest randomized clinical trial of TXA for acute GI bleed
- Randomization was well done with patients, caregivers, and outcome assessors blinded to allocation
- Patients were evenly balanced between groups (see table 1)
- Used a modified intention-to-treat analysis, excluding patients who did not receive a dose of the allocated treatment and those where outcome data on death were unavailable
- Excellent follow up: Obtained primary outcome data for all but 3 patients in the TXA group
- Used clinical inclusion criteria with a full range of GIB presentations which increases usefulness of study clinically
- Some patients received antifibrinolytics outside the protocol, but the authors reexamined the results with exclusion of these patients and found no difference in the results
Limitations:
- Sample size calculation was initially based on all-cause mortality as the primary outcome, however halfway through the trial, it was realized over half of all deaths were due to non-bleeding causes. Therefore, the primary outcome was changed to bleeding with 5d of randomization. This gave the study the power to detect a clinically important 25% relative reduction or 1% absolute reduction in death due to bleeding from 4% to 3% (Authors assumed a 4% risk of death due to bleeding).
- The majority of patients included in this trial had variceal bleeding due to liver disease and accounted for ≈75% of deaths. Additionally, increased risk of venous thromboembolic events with TXA appeared to be more pronounced in patients with liver disease
- Only 9% of patients in the study were on anticoagulants. The applicability of the data to that group is more uncertain
Discussion:
- Time from onset to randomization ≤3 hours was only achieved in 16% of patients in both groups (Most patients were >8 hours in approximately 57% of both groups). However, looking at the subgroup of patients in the ≤3hr vs >3hr since time of onset there was no statistical difference in the primary outcome.
- As opposed to trauma and postpartum hemorrhage the exact timing of GIB is more difficult. Additionally, most patients presented more than 3h after bleeding onset. All of which could result in lack of benefit in TXA compared to placebo. As a matter of fact the time from onset to administration of either TXA or placebo was on average >20hrs.
- Most patients had a systolic blood pressure ≥90mmHg (87% of patients)
- Rockall score was used to predict mortality rates in patients, with a higher score predicting a higher mortality. This was a pretty sick group of patients with the breakdown of patients being:
- Score 1 – 2 (Mortality 2.4 to 5.6%): 24%
- Score 3 – 4 (Mortality 11 to 24.6%): 38%
- Score 5 – 7 (Mortality 39.6 to 50%): 38%
- The dose of TXA used in this trial was higher, but duration of treatment was longer (4g over 24h) compared to previous studies on trauma (2g over 8h) or postpartum hemorrhage (1g bolus with a repeat 1g dose if bleeding continued)
- The half-life of TXA is 2 hours and there is a higher risk of rebleeding in the first 24 hours, therefore, the authors chose 24hours of treatment as they wanted to cover the high-risk period of time for rebleeding
Author Conclusion: “We found that tranexamic acid did not reduce death from gastrointestinal bleeding. On the basis of our results, tranexamic acid should not be used for the treatment of gastrointestinal bleeding outside the context of a randomized trial.”
Clinical Take Home Point: This is a very well done, large, multicenter randomized controlled trial of TXA vs placebo for acute GIB. The results demonstrate no benefit of giving TXA on 5d mortality in patients with acute GIB and a small signal of harm with increased VTE and seizures. TXA should not be recommended at this time for patients with acute GIB.
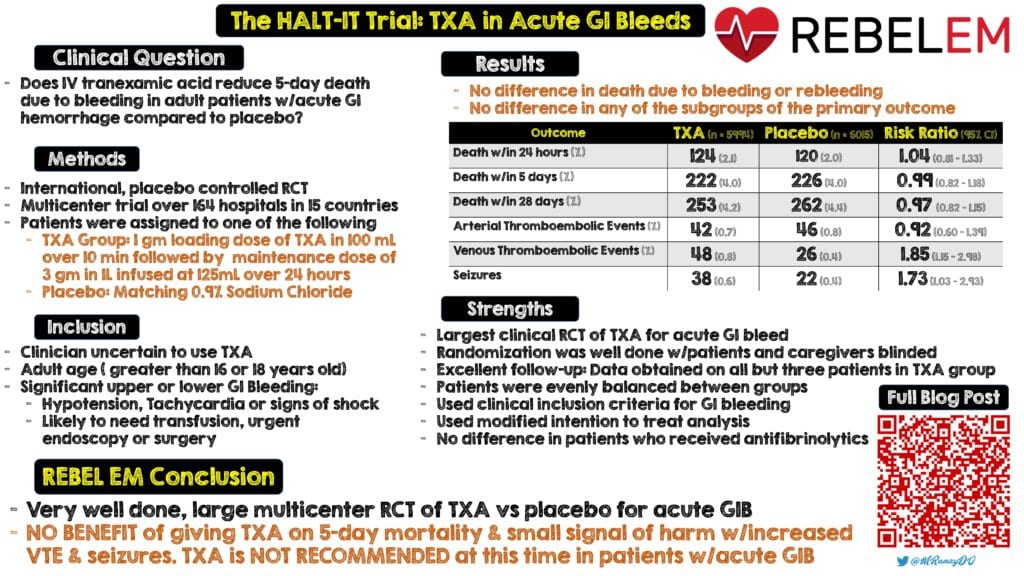
Infographic by Mark Ramzy, DO (Twitter: @MRamzyDO)
References:
- The HALT-IT Trial Collaborators. Effects of High-Dose 24-h Infusion of Tranexamic Acid on Death and Thromboembolic Events in Patients with Acute Gastrointestinal Bleeding (HALT-IT): An International Randomised, Double-Blind, Placebo-Controlled Trial. Lancet 2020. [Epub Ahead of Print]
- Gluud LL et al. Tranexamic Acid for Upper Gastrointestinal Bleeding. Cochrane Database Syst Rev 2012. PMID: 25414987
For More Thoughts on This Topic Checkout:
- The Resus Room:XA in GI Bleeds
- First10EM: TXA for GI Bleeds – No Benefit (The HALT-IT Trial)
- St. Emlyn’s: Halt! It’s Not Time for TXA! Or is it? HALT-IT Results
- The Bottom Line: HALT-IT
- The SGEM: SGEM#301 – You Can’t Stop GI Bleeds with TXA
Post Peer Reviewed By: Anand Swaminathan, MD (Twitter: @EMSwami)
The post REBEL Cast Ep85: The HALT-IT Trial – TXA in Acute GI Bleeds appeared first on REBEL EM - Emergency Medicine Blog.