Background: There are three randomized clinical trials now published on remdesivir in the treatment of COVID-19 pneumonia (RCT 1, RCT 2, & RCT 3). The 1st trial, performed in China, was terminated early due the lack of patients to enroll and, as a result, did not give strong recommendations. The 2nd trial (ACTT-1) showed a statistically significant 4-day reduction in time to recovery. However, it was also terminated early due to an interim analysis, which meant we do not have outcomes on 30% of patients enrolled. Finally, the 3rd RCT compared a 5-day to a 10-day course of remdesivir and showed no difference in outcomes with more acute kidney injury in the 10-day course. All of these trials have significant issues leaving clinicians unsure of the efficacy of the drug, when to administer it, how long to give it for and, in which patient group it should be given. We now have our 4th RCT of remdesivir evaluating the efficacy and adverse events of remdesivir administered for 5- or 10-days vs standard care in hospitalized patients with moderate COVID-19.
Background: There are three randomized clinical trials now published on remdesivir in the treatment of COVID-19 pneumonia (RCT 1, RCT 2, & RCT 3). The 1st trial, performed in China, was terminated early due the lack of patients to enroll and, as a result, did not give strong recommendations. The 2nd trial (ACTT-1) showed a statistically significant 4-day reduction in time to recovery. However, it was also terminated early due to an interim analysis, which meant we do not have outcomes on 30% of patients enrolled. Finally, the 3rd RCT compared a 5-day to a 10-day course of remdesivir and showed no difference in outcomes with more acute kidney injury in the 10-day course. All of these trials have significant issues leaving clinicians unsure of the efficacy of the drug, when to administer it, how long to give it for and, in which patient group it should be given. We now have our 4th RCT of remdesivir evaluating the efficacy and adverse events of remdesivir administered for 5- or 10-days vs standard care in hospitalized patients with moderate COVID-19.
Paper: Spinner CD et al. Effect of Remdesivir vs Standard Care on Clinical Status at 11 Days in Patients With Moderate COVID-19: A Randomized Clinical Trial. JAMA 2020. PMID: 32821939
Clinical Question: What is the efficacy of 5 or 10 days of remdesivir compared with standard care on clinical status at day 11 after initiation of treatment in patients with moderate COVID-19 pneumonia?
What They Did:
- Randomized, open-label, phase III trial of patients with moderate COVID-19 pneumonia
- Pulmonary infiltrates
- Room air oxygen saturation >94%
- 105 hospitals in the US, Europe, and Asia
- Patients randomized 1:1:1 to:
- 10-day course of remdesivir
- 5-day course of remdesivir
- Standard care
- Remdesivir dosed at 200mg IV on day 1, followed by 100mg qD
Outcomes:
-
Primary: Clinical status on day 11 on a 7-point ordinal scale
- Category 1 = Death
- Category 2 = Hospitalized, requiring invasive mechanical ventilation or ECMO
- Category 3 = Hospitalized, requiring NIV or HFNC
- Category 4 = Hospitalized, requiring low flow supplemental oxygen
- Category 5 = Hospitalized, not requiring supplemental oxygen but requiring ongoing medical care (related or not to COVID-19)
- Category 6 = Hospitalized, not requiring supplemental oxygen or ongoing medical care
- Category 7 = Discharged
- An odds ratio (OR) >1 indicates a difference in clinical status distribution toward category 7 for remdesivir vs standard care group
-
Secondary:
- Proportion of patients with adverse events
Inclusion:
- Hospitalized patients with SARS-CoV-2 infection within 4 days of randomization
- Moderate COVID-19 pneumonia
- Any radiographic evidence of pulmonary infiltrates
- O2 saturation >94% on room air
Exclusion:
- ALT or AST >5x the upper limit of normal
- Creatinine clearance <50mL/min
- Pregnant or Breastfeeding
- Known hypersensitivity to study drug
- Requiring mechanical ventilation at screening
Results:
- 596 patients randomized
- 584 began study and received remdesivir or standard care
- 533 (91%) completed the trial
- Median length of treatment
- 5-day remdesivir group: 5 days
- 10-day remdesivir group: 6 days
- Median duration of symptoms before 1st dose of remdesivir was ≈8 days in all groups
- 76% of patients completed therapy in the 5d group and 38% in the 10d group
-
Odds of Better Clinical Status at Day 11 (Primary Outcome):
- 5 Day Remdesivir vs Standard Care: OR 1.65; 95% CI 1.09 – 2.48; p = 0.02
- 10 Day Remdesivir vs Standard Care: Not statistically different p = 0.18
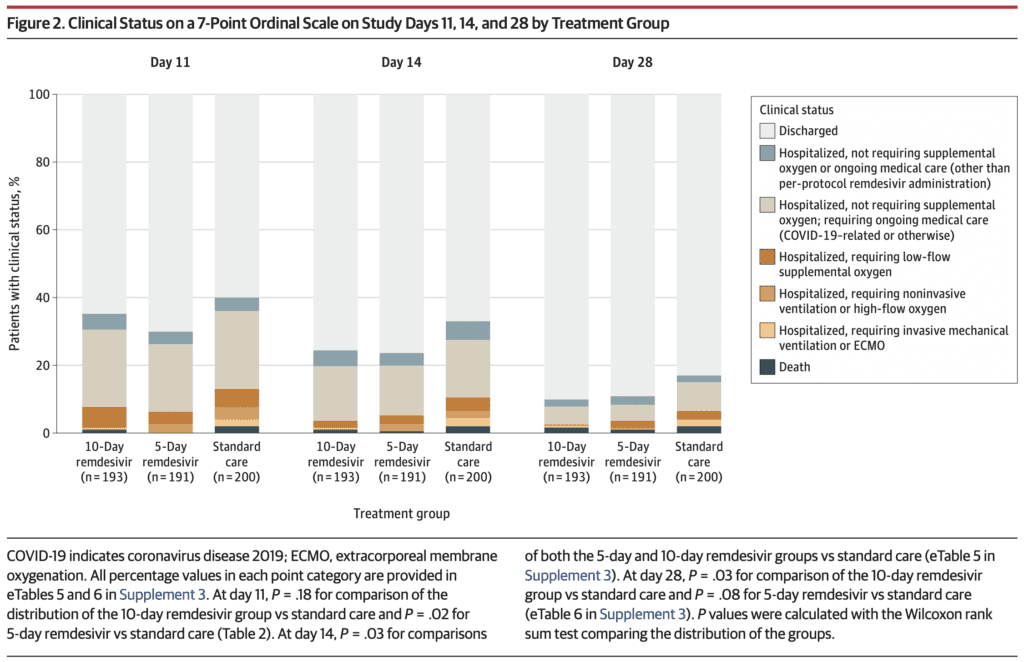
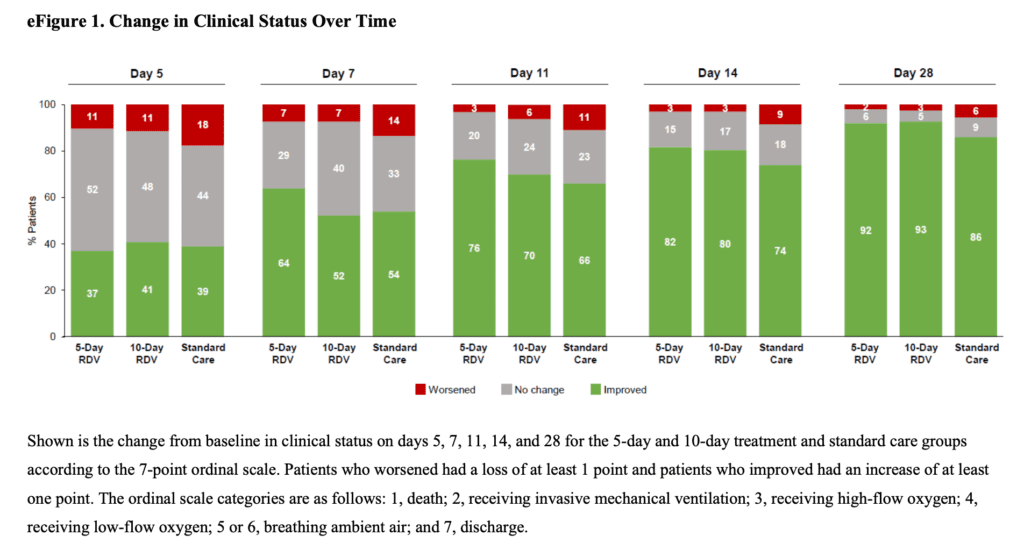
- No difference in remdesivir and standard care groups in:
- Duration of oxygen therapy or hospitalization
- 28-day all-cause mortality
- Adverse Events:
- 5d Remdesivir: 51%
- 10d Remdesivir: 59%
- Standard Care: 47%
- Difference not statistically significant between 5d and standard care (Difference 4.8%; 95% CI -5.2% to 14.7%; p = 0.36)
- Difference was statistically significant between 10d and standard care (Difference 12.0%; 95% CI 1.6% to 21.8%; p = 0.02)
- Adverse events more common in remdesivir groups compared to standard care:
- Nausea
- Hypokalemia
- Headache
- Serious adverse events were less common in the remdesivir groups (5% in both) than standard care group (9%)
- Creatinine Clearance Decrease:
- 5d Remdesivir: 15%
- 10d Remdesivir: 26%
- Standard Care: 30%
Strengths:
- Randomized clinical trial asking an important clinical question
- Groups were well balanced at baseline including demographics and disease characteristics
Limitations:
- Treatment was open label due to an insufficient number of placebo-containing vials. Absence of blinding brings significant bias to these results
- The primary endpoint of the study was altered from proportion of patients discharged by day 14 to assessment of clinical status on a 7-point ordinal scale by day 11 (alteration was made prior to any data collection but, the original primary endpoint is more objective and, more clinically relevant)
- The original protocol allowed use of other agents with presumed activity against SARS-CoV-2 based on local standard care and although disallowed in a subsequent amendment, some patients had already received the other concurrent therapies
- Patients not stratified by site at enrollment, which could lead to imbalances in patient care and discharge practices
- We have no data on virologic effects (i.e. viral load) of remdesivir on SARS-CoV-2
- Additional labs may have aided in identifying predictors of outcomes
- Ordinal scale is not ideal for detecting differences in patients with moderate COVID-19
- Nonconsecutive enrollment of patients
- Standard care wasn’t uniform. Many patients in the standard care arm received other meds in comparison to the remdesivir groups. HCQ is associated with worse outcomes so this may have biased the results
- Placebo group slightly sicker at baseline
Discussion:
- Original endpoint is more important (proportion of patients discharge by day 14) rather than the assessment of clinical status on a 7-point ordinal scale by day 11. What we want to know is the proportion of patients we can get home or survive. Great if patient is better but, better doesn’t mean patients were discharge home
- Only 15 to 19% of patients received steroids as concomitant therapy in this study
- Patients in the 10d arm of remdesivir only received a median of 6d of treatment, which is similar to 5d of treatment seen in the 5d arm. This is important because, it brings up the question of whether the effect seen in the 5-day arm is just random chance. If it wasn’t, you would expect to see benefit in the 10-day arm since most patients got about 5 to 6 days of the drug.
- Discharge decisions may have been influenced by the assigned duration of remdesivir therapy which is one reason we may have not seen any benefit in the 10d remdesivir group compared to standard care. This is evidenced by the rates of discharge increasing the day after the end of the dosing in both groups
- Now we have 4 RCTs of remdesivir in hospitalized patients with differing results. This raises the question of whether the positive results are statistical noise due to study design/patient populations or the drug is just not as good as we think
- In the ACTT-1 trial remdesivir was of no benefit in the really sick (HFNC, NIV, IMV, and ECMO) and completely useless in patients that didn’t need O2. Combined with these results, it seems the patients most likely to benefit are the ones requiring low flow O2 (i.e. Not too early in disease and not too late in disease)
- Using an ordinal scale can be problematic as each change in the ordinal scale is not equivalent (i.e. no oxygen to low flow oxygen vs requiring mechanical ventilation to death). The distribution of differences is not proportional in COVID-19 therefore making it clinically difficult to quantify the net benefit on one end of the scale vs another
Author Conclusion: “Among patients with moderate COVID-19, those randomized to a 10-day course of remdesivir did not have a statistically significant difference in clinical status compared with standard care at 11 days after initiation of treatment. Patients randomized to a 5-day course of remdesivir had a statistically significant difference in clinical status compared with standard care, but the difference was of uncertain clinical importance.”
Clinical Take Home Point: Combining all the evidence we have thus far, remdesivir is far from a savior in COVID-19. However, we have some signal that the drug may be beneficial in a very select group of patients: earlier in disease with moderate severity (i.e. low flow oxygen). There are still some big questions left unknown in terms of optimal use of remdesivir:
- What is the optimal patient population?
- What is the optimal duration of therapy?
- What is the effect on discrete clinical outcomes?
- What is the effect of the drug if given in conjunction with dexamethasone or other corticosteroids?
References:
- Spinner CD et al. Effect of Remdesivir vs Standard Care on Clinical Status at 11 Days in Patients With Moderate COVID-19: A Randomized Clinical Trial. JAMA 2020. PMID: 32821939
- McCreary EK et al. Efficacy of Remdesivir in COVID-19. JAMA 2020. PMID: 32821934
For More Thoughts on This Topic Checkout:
- REBEL EM: COVID-19 – Remdesivir RCT #3 (5d vs 10d)
- REBEL EM: COVID-19 – Two More Trials Just Published on Remdesivir
- REBEL EM: Remdesivir ACTT-1 – Adaptive COVID-19 Treatment Trial Part 1
Post Peer Reviewed By: Anand Swaminathan, MD (Twitter: @EMSwami)
The post Remdesivir in Moderate COVID-19 appeared first on REBEL EM - Emergency Medicine Blog.