 Background: CT coronary angiography (CTCA) is a relatively new technology that has gained popularity over the past few years in evaluating patients presenting with chest pain. CTCA is an anatomic test that has been shown to increase downstream testing and increase healthcare costs but its impact on patient-oriented benefit has been questioned. Early concerns of CTCA including poor image quality in the obese and high levels of radiation exposure have been mitigated by improved technology.
Background: CT coronary angiography (CTCA) is a relatively new technology that has gained popularity over the past few years in evaluating patients presenting with chest pain. CTCA is an anatomic test that has been shown to increase downstream testing and increase healthcare costs but its impact on patient-oriented benefit has been questioned. Early concerns of CTCA including poor image quality in the obese and high levels of radiation exposure have been mitigated by improved technology.
Another trial, called PROMISE, also evaluated anatomic CCTA vs functional stress testing in greater than 10,000 patients with symptomatic chest pain with suspected CAD. In this study an initial strategy of CCTA was not associated with better clinical outcomes compared to functional testing over a median follow-up period of two years, and it was also associated with higher radiation exposure and downstream testing.
In this post we will cover the original SCOT-HEART trial published in 2015 [1] and the 5 year follow up of the original SCOT-HEART trial [2].
Original SCOT-HEART Trial [1]
What They Did: This was a prospective open-label, parallel-group, multicenter, randomized trial of patients, aged 18 – 75 years of age referred for assessment of suspected angina due to coronary heart disease from 12 cardiology chest pain clinics across Scotland. Patients were randomized in a 1:1 fashion into standard care plus CCTA (CCTA arm) vs standard care alone (Standard care arm).
Outcomes:
- Primary: Proportion of patients diagnosed with angina pectoris secondary to coronary heart disease at 6 weeks
- Long-Term Outcomes:
- Death
- Myocardial Infarction
- Coronary Revascularization procedures
- Admission to hospital for chest pain episodes
- Cerebrovascular disease
- Peripheral vascular disease
- Safety Outcomes:
- Dose-length product and effective radiation dose calculated (0.014mSv/mGy x cm conversion factor)
- Contrast reaction
- Renal impairment
- Vasovagal response
Inclusion:
- Age 18 – 75 years
- Referred by primary care physician to dedicated cardiology chest pain clinic
- Suspected angina due to coronary heart disease
Exclusion:
- Inability to undergo CT scanning
- Renal failure (serum Cr >250umol/L or eGFR <30mL/min)
- Previous recruitment to the trial
- Major allergy to iodinated contrast media
- Inability to give informed consent
- Known pregnancy
- Acute coronary syndrome within 3 months
Results:
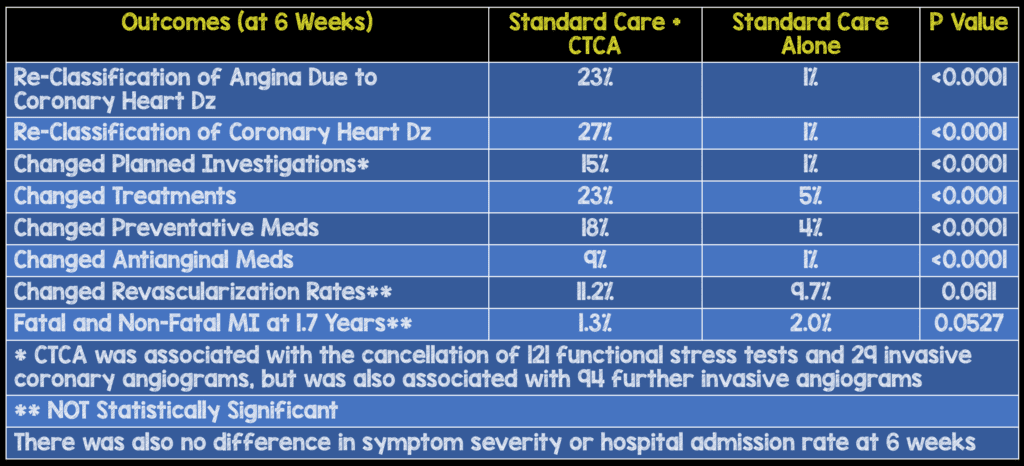
- 4,146 (42%) patients out of 9,849 patients who had been referred for assessment of suspected angina due to coronary heart disease
- After clinic consultation, 1778 participants were randomly assigned to CTCA
- All adverse reactions to CTCA were mild and self-limiting, with no cases of anaphylaxis or renal failure
- Median radiation dose was 4.1 mSv
Strengths:
- Large, Multicenter, randomized trial
- Groups were balanced at baseline in terms of age, BMI, and comorbid conditions
- All CTCAs were assessed by at least two accredited assessors and the first 40 scans from each imaging site underwent independent central validation to ensure consistency of approach
- Assessors reported the presence of coronary heart disease with excellent intra-observer agreement of 95% and inter-observer agreement of 91%
- The funder of the study had no role in the trial conduct including data collection, analysis, interpretation, or writing of the manuscript
- Allowed unrestricted use of further stress imaging in keeping with clinician choice and routine practice to explore the effect of the addition of CTCA as opposed to doing a head-to-head comparison with other diagnostic approaches
- Focused on the effect of CTCA on patient-centered and clinician-centered outcomes rather than comparing diagnostic accuracy between imaging modalities or anatomic versus functional testing
- Assessed the effect of CTCA on both short-term and long-term outcomes to define the impact of CTCA to routine clinical practice
Limitations:
- Cardiology chest pain clinic study, not a study of acute chest pain (i.e. stable angina not unstable angina)
- The overall absolute event rate during a median of 1.7 years of follow-up was very low at 2%
- Open label trial, meaning clinicians may favor CTCA or functional tests, which could have affected their decision making
- Alterations in patient management based on the CTCA may have taken more than 6 weeks to be effectively implemented in some patients. Therefore, we cannot make any conclusions on anginal symptoms
- The cost-effectiveness of CTCA and downstream health-care resource utilization was not assessed
- Patients >75 years were excluded from this study
Discussion:
- In the standard care arm, an ASSIGN score, a validated Scottish cardiovascular risk score that incorporates social deprivation and family history of cardiovascular disease was used to calculate cardiovascular risk
- CT scans were done with either a 64 slice detector row scanner or 320 slice detectors, the majority of which were the 320 slice scanners (75.5%)
- Obstructive coronary artery disease was defined as a luminal stenosis of >70% in one or more major epicardial vessels or >50% stenosis in the left main stem.
- Luminal cross-sectional area stenoses were classified in the following manner:
- Normal <10%
- Mild non-obstructive 10 – 49%
- Moderate non-obstructive 50 – 70%
- Obstructive >70%
- To no surprise clinicians taking care of the patients felt that CTCA increased certainty (RR 2.56) and frequency (RR 1.09) of the diagnosis of coronary heart disease at 6 weeks compared to standard care
- CTCA changed the classification of angina due to coronary heart disease in one in four patients. This is also evident in the modest net increase in the use of invasive coronary angiography (Although, this was not statistically significant in this study).
- An important point that cannot be emphasized enough is with an increased change in diagnosis, the overall preventative medications were increased, which by themselves could be responsible for improved patient oriented outcomes, while antianginal treatments were decreased.
- This increased diagnostic certainty is mostly a clinician benefit, though does provide some downstream advantage to patients, who may see more individualized and appropriate medication manipulation, with decreased exposure to unnecessary medications and their side effects.
- I often see coronary artery disease and acute coronary syndrome confounded. It should be clarified that this is a patient population of coronary artery disease and coronary revascularization is not associated with improved outcomes in patients with chronic stable coronary heart disease, but it is associated with improved outcomes in those with acute coronary syndromes.
Author Conclusion: “In patients with suspected angina due to coronary heart disease, CTCA clarifies the diagnosis, enables targeting of interventions, and might reduce the future risk of myocardial infarction.”
Follow-Up SCOT-HEART Trial [2]
What They Did: This was a 5 year follow up to the original SCOT-HEART trial which was an open-label, multicenter, parallel-group, multicenter trial of patients aged 18 – 75 years of age with stable chest pain who had been referred to a 12 cardiology clinics in Scotland for evaluation to standard care plus CTCA vs standard care alone.
Outcomes:
- Primary: Death from coronary heart disease or nonfatal myocardial infarction at 5 years
- Clinical Endpoints:
- Death (cardiovascular death, noncardiovascular death, death from coronary heart disease, and death from any cause)
- Myocardial infarction
- Stroke
Inclusion and Exclusion:
- Same as original SCOT-HEART trial
Results:
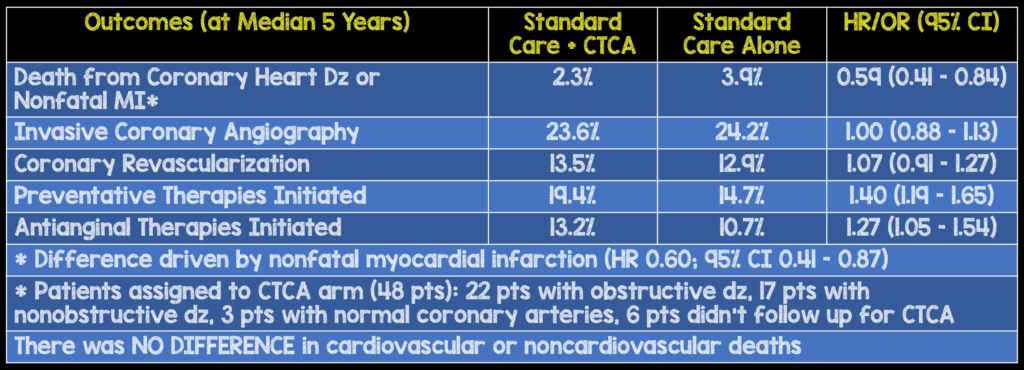
- Median duration of follow-up was 4.8 years (Range: 3 – 7 years)
Strengths:
- Large, multicenter, randomized trial
- Funders had no role in the design or conduct of the trial or in the collection, analysis, or reporting of data
- Performed a 12 month analysis which was able to distinguish the immediate effects of CTCA from the longer-term consequences.
Limitations:
- There was no formal event adjudication
- Low cardiovascular event rates (3.1%)
- Again, this was an open-label trial, and ascertainment bias is inherent to the trial design. Ascertainment bias is a systematic distortion in measuring the true frequency of a phenomenon due to the way in which the data is collected. This is related to sampling and selection bias
- Lifestyle modification alterations were not recorded during follow-up
- Cardiovascular risk thresholds for the initiation of preventative therapies have fallen over the past few years, since this trial was completed and it is unclear whether the benefits of CTCA will be maintained with these lower thresholds—though, as noted in the initial SCOT-HEART analysis, ostensibly increased diagnostic certainty modulates patient exposure to preventative and anti-anginal therapies.
- The benefit of CTCA with respect to the rate of death from coronary heart disease and nonfatal myocardial infarction was only 1.6% lower than the rate with standard therapy and is rather modest for increased downstream testing and healthcare cost.
Discussion:
- Rates of invasive coronary angiography and coronary revascularization were higher in the CTCA group vs the standard card group in the first few months of follow up, but the overall rates were similar at 5 years. This suggests that use of CTCA resulted in more appropriate use of preventative therapies in the CTCA group, and this change in management resulted in fewer clinical events compared to standard care.
- The authors also conclude that, CTCA resulted in a higher rate of detection of obstructive coronary heart disease, and this detection led to more appropriate use of coronary angiography and coronary revascularization. But it is important to realize that these patients were also more likely to receive appropriate preventative therapies and may have greater motivation to implement healthy lifestyle modifications, a more likely driver of benefit, particularly given results from the recent ORBITA trial. Additionally, physicians were encouraged to initiate secondary prevention strategies in patients with nonobstructive coronary artery disease by trial investigators which is not routinely done in general practice.
- It is likely that for those in whom CTCA demonstrated no evidence of obstructive CAD, minor troponin elevations were classified differently than in those who did not undergo CTCA, who would therefore be more likely designated as NSTEMI. Such “shell game” reclassification may account for a large proportion of the decreased non-fatal Mis which drive the primary endpoint.
- In the PROMISE trial, preventative therapies were not mandated, and two thirds of subsequent cardiac events occurred in patients with nonobstructive disease
- Changes in outcomes occurred only once treatment interventions directed by CTCA findings were initiated. It is possible that immediate reductions in events were mediated through the use of aspirin alone and not aspirin and coronary revascularization procedures. The longer term benefits are most likely attributable to lifestyle modification and addition of other preventative therapies, such as statins and not coronary revascularization.
- One argument for CTCA is that although it does increase over testing, it identifies patients with coronary disease better than current scoring systems, which have poor predictive accuracy
- Finally, the authors of this trial conclude that 63 patients with stable chest pain would need to be referred for CTCA to prevent 1 fatal or nonfatal myocardial infarction over the course of 5 years.
Author Conclusion:“In this trial, the use of CTCA in addition to standard care in patients with stable chest pain resulted in a significantly lower rate of death from coronary heart disease or nonfatal myocardial infarction at 5 years than standard care alone, without resulting in significantly higher rate of coronary angiography or coronary revascularization.”
Clinical Take Home Point: Because CTCA is an anatomic test, it appears to increase the certainty and frequency of coronary heart disease identification, which in turn changes treatment with preventative and anti-anginal medications, but also leads to more coronary angiography and revascularization in the short term (12months). With the newer AHA recommendations for lowered thresholds of preventative medication initiation, since the publication of the original SCOT-HEART study, it is not clear if CTCA would still result in improved long term outcomes. Additionally, with SCOT-HEART’s primary 5-year endpoint being driven by decreased non-fatal MI, an outcome that may be differently adjudicated based on the data ascertained from CTCA, even this improvement is suspect. Generally, with the publication of SCOT-HEART, the addition of CTCA to standard care can be definitively determined to have little benefit except to mitigate diagnostic uncertainty—a clinician-oriented benefit with minimal patient value. The only question that remains unanswered now is, does the initiation of preventative therapies (aspirin, statins, lifestyle modifications), coronary revascularization, or a combination of both strategies improve long-term patient oriented outcomes in patients with stable chest pain due to coronary heart disease?
References:
- The SCOT-HEART Investigators. CT Coronary Angiography in Patients with Suspected Angina due to Coronary Heart Disease (SCOT-HEART): An Open-Label, Parallel-Group, Multicentre Trial. Lancet 2015. PMID: 25788230
- The SCOT-HEART Investigators. Coronary CT Angiography and 5-Year Risk of Myocardial Infarction. NEJM 2018. PMID: 30145934
Post Peer Reviewed By: Rick Pescatore, DO (Twitter: @Rick_Pescatore)
The post SCOT-HEART: CCTA Decreases Long-Term Risk of MI and Death? appeared first on REBEL EM - Emergency Medicine Blog.
