
 Background: Acute Aortic Syndromes (AAS) are life threatening cardiovascular emergencies that are the bane of every emergency physician’s existence. They are diagnostic challenges due to the clinical presentation being highly non-specific. Computed tomography angiography (CTA), Transesophageal Echocardiography (TEE), and Magnetic Resonance Angiography (MRA) can help accurately diagnose AAS. CTA exposes patients to radiation and large doses of intravenous contrast, neither of which is a good enough reason to skip the test in patients you think may have a dissection, but certainly not something we want to do to all patients coming to the ED with chest pain. TEE and MRA may not be available or able to be performed in a timely manner. Having a clinical algorithm that can help physicians reduce misdiagnosis and at the same time avoid over-testing are lacking.
Background: Acute Aortic Syndromes (AAS) are life threatening cardiovascular emergencies that are the bane of every emergency physician’s existence. They are diagnostic challenges due to the clinical presentation being highly non-specific. Computed tomography angiography (CTA), Transesophageal Echocardiography (TEE), and Magnetic Resonance Angiography (MRA) can help accurately diagnose AAS. CTA exposes patients to radiation and large doses of intravenous contrast, neither of which is a good enough reason to skip the test in patients you think may have a dissection, but certainly not something we want to do to all patients coming to the ED with chest pain. TEE and MRA may not be available or able to be performed in a timely manner. Having a clinical algorithm that can help physicians reduce misdiagnosis and at the same time avoid over-testing are lacking.
What They Did:
- Multicenter, prospective observational study
- 6 Hospitals in 4 countries
- Use of the Aortic Dissection Detection Risk Score (ADD-RS) of ≤1 + Negative D-Dimer (DD) for ruling out AAS
Outcomes:
-
Primary: Failure rate of ADD-RS and D-Dimer neg strategy for ruling out AAS
- Calculation: AAS diagnoses/ Number of Patients with Negative DD Within in a Risk Category
-
Secondary: Efficiency in Ruling-Out AAS
- Calculation: Number of Patients with Negative DD Within a Risk Category/Number of Enrolled Patients
Inclusion:
- Consecutive patients ≥18 years of age, with any 1 or more of the following symptoms, dating ≤14 days:
- Chest/Abdominal/Back Pain
- Syncope
- Perfusion Deficit
- Acute Aortic syndrome in Differential Diagnosis
Exclusion:
- Primary trauma
- Unwillingness or inadequacy to participate in the study
Definitions:
- Pre-Test Probability Assessment (ADD-RS): Based on 12 risk markers classified in 3 categories
- Calculated by number of categories (0 – 3) where at least one risk marker was present
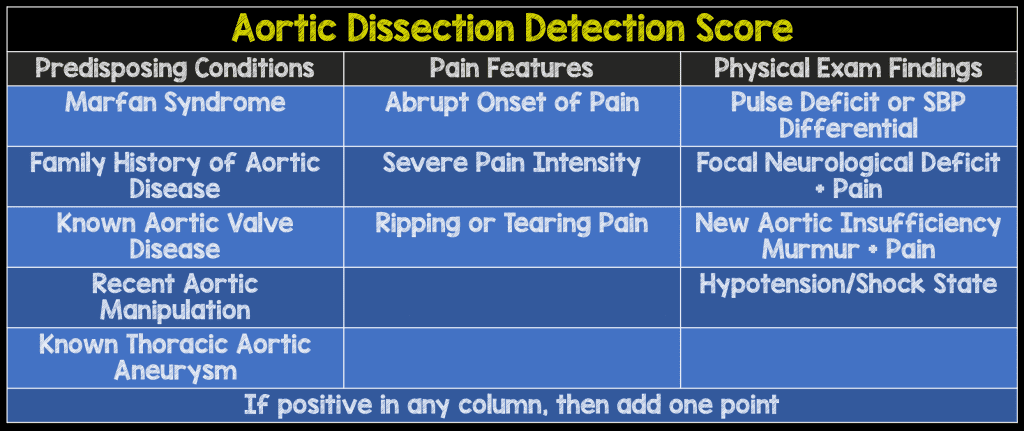
- D-Dimer (DD): Defined as negative if <500ng/mL fibrinogen equivalent units
- Conclusive Imaging Used for Diagnosis of AAS: CTA, TEE, and/or MRA
Results:
- 1850 patients analyzed
- 438 (24%) patients had ADD-RS = 0
- 1071 (58%) patients had ADD-RS = 1
- 341 (18%) patients had ADD-RS > 1
- 241 (13%) patients had AAS
- Positive DD (≥500 mg/mL) for Diagnosis for AAS
- 813 (43.9%) of patients
- 585 (38.8%) patients had ADD-RS≤1
- 228 (66.9%) patients had ADD-RS>1
- 813 (43.9%) of patients
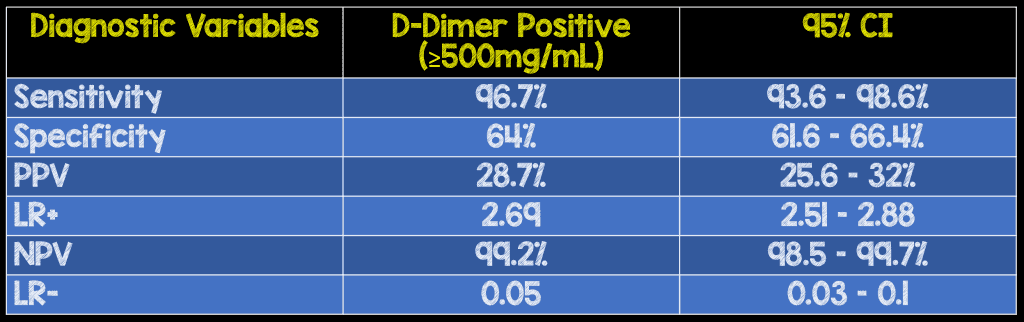
- 8 patients with AAS had a negative DD
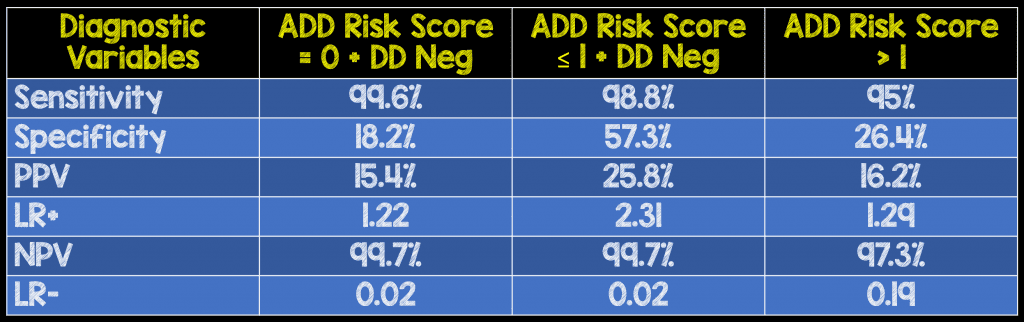
Critical Results:
Strengths:
- Asks an important clinical question
- Multicenter study increases generalizability
- First study to really look at how to implement d-dimer into a diagnostic algorithm
- Addresses the need issue from ACEP to not incorporate d-dimer until a risk stratification tool had been developed
Limitations:
- Observational study with many confounders
- Physicians not blinded to items for pre-test probability assessment or the DD test results, potentially causing bias in this study
- Although symptoms triggering screening were pre-specified, entry criterion were provider determined
- Half the patients in this study did not have conclusive imaging and their case follow up was based on 14-day clinical follow up data only
- Unclear if 14 day follow up is an adequate time period
- There is no comparison to clinical gestalt
- Rate of AAD is pretty high (i.e. 13%), making it unclear how this would work in lower risk groups
- Only use one d-dimer assay, that may not be available at all institutions
- No discussion of age-adjustment of d-dimer
Discussion:
- In this paper, the authors suggest the following algorithm:
- ADD-RS>1, regardless of DD should proceed to CTA
- ADD-RS = 0 or ≤ 1 + DD neg are potentially ruled out for AAS
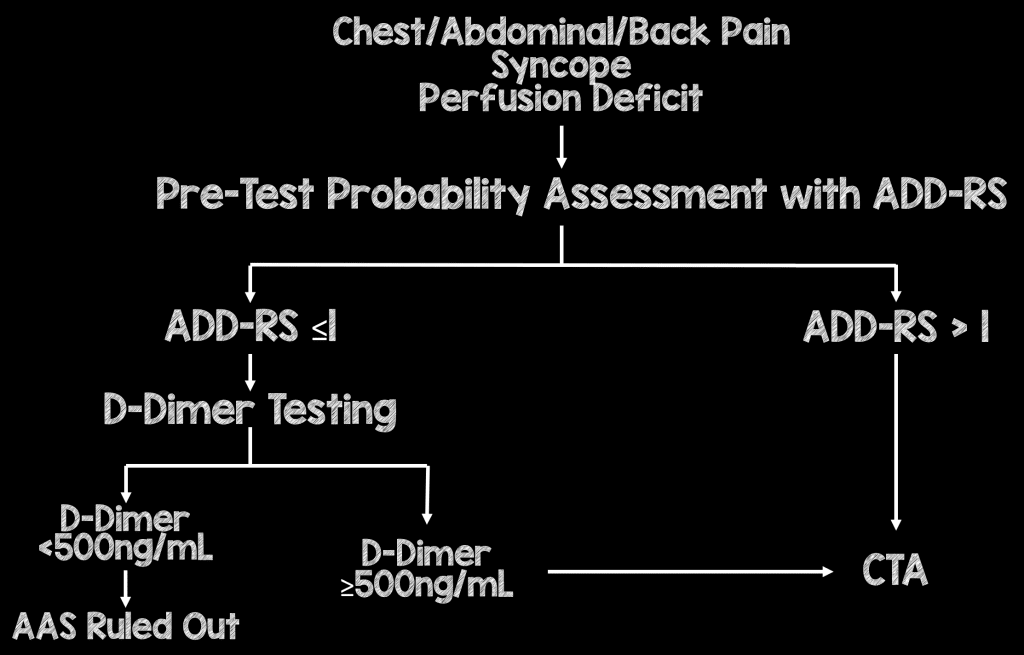
This strategy will miss around 1 in 300 cases of AAS
Author Conclusion: “Integration of ADD-RS (both = 0 or ≤ 1) with DD may be considered to standardize diagnostic rule-out of AAS.”
Clinical Take Home Point:
This is a novel clinical strategy in evaluating patients with the potential of Acute Aortic Syndromes (AAS), but still requires external validation, for reproducibility and comparison to overall clinical gestalt before implementation into clinical practice.
References:
- Nazerian et al. Diagnostic Accuracy of the Aortic Dissection Detection Risk Score Plus D-Dimer for Acute Aortic Syndromes: The ADvISED Prospective Multicenter Study. Circulation 2017. PMID: 29030346
Post Peer Reviewed By: Anand Swaminathan (Twitter: @EMSwami)
The post The ADvISED Trial: A Novel Clinical Algorithm for the Diagnosis of Acute Aortic Syndromes appeared first on REBEL EM - Emergency Medicine Blog.
