

Background: COVID-19 infection increases the risk of thrombosis due to multiple factors.(Rico-Mesa, 2020). Over the last 2 years, researchers have published 12 RCTs investigating various anticoagulation strategies among patients diagnosed with COVID-19 in multiple clinical settings. To date, only a multiplatform trial in noncritically ill patients [Link is here], the HEP-COVID trial [Link is here], and the MICHELLE trial [Link is here] have shown a benefit.
Severely ill patients with COVID-19 in the intensive care setting have an increased risk of thromboembolism, with an incidence reported as high as 31% (Klok, 2020). It remains unclear what the optimal prophylactic anticoagulation strategy is in critically ill patients.
Article: Sadeghipour P, Talasaz AH, et al. Effect of Intermediate-Dose vs. Standard-Dose Prophylactic Anticoagulation on Thrombotic Events, Extracorporeal Membrane Oxygenation Treatment, or Mortality Among Patients With COVID-19 Admitted to the Intensive Care Unit: The INSPIRATION Randomized Clinical Trial. JAMA. 2021;325(16):1620-1630. PMID: 33734299
Clinical Question: Does intermediate-dose anticoagulation improve clinical outcomes compared to standard prophylactic anticoagulation in patients with COVID-19 treated in the intensive care unit?
What They Did:
- Open-label, multicenter, randomized trial with a 2×2 factorial design with blinded adjudication
- Patients were enrolled from 10 academic centers in Iran
- Patients were screened between July 29, 2020, and November 19, 2020.
-
Participants were randomized in a 1:1 ratio to receive an intermediate dose of enoxaparin (1 mg/kg daily) or the standard prophylactic dose of enoxaparin (40 mg daily) for 30 days.
- Patients with severe kidney disease were given unfractionated heparin.
- Modifications were made for both groups based on body weight and creatinine clearance.
- Investigators completed weekly phone interviews with patients and follow-up after 30 days.
Population:
-
Inclusion Criteria:
- Patients >18 years old admitted to the ICU with a positive COVID-19 PCR test within 7 days of hospitalization
-
Exclusion Criteria:
-
- Life expectancy less than 24 hours
- Other indications for therapeutic anticoagulation
- Weight less than 40 kg
- Pregnant
- History of heparin-induced thrombocytopenia
- Platelet count less than 50 ×1000/µL
- Overt bleeding
-
Intervention
- 1 mg/kg enoxaparin daily for 30 days (intermediate dose)
Control:
- 40 mg enoxaparin daily for 30 days (standard prophylactic dose)
Outcomes:
-
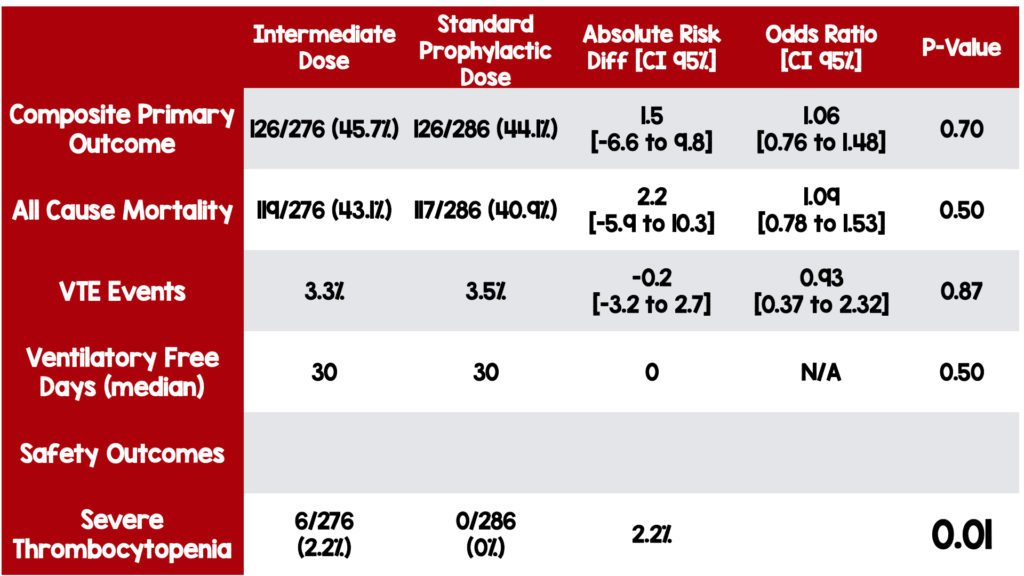
Primary Outcome (Composite outcome at the end of 30 days):
- Venous thromboembolism
- Arterial thrombosis
- Treatment with extracorporeal membrane oxygenation (ECMO)
- All-cause mortality
-
Secondary Outcomes:
- All-cause mortality
- Venous thromboembolism
- Ventilator free days
-
Principal Safety Outcomes:
- Major bleeding according to the Bleeding Academic Research Consortium criteria.
- Thrombocytopenia
Results:
- 1692 patients screened for eligibility
-
600 patients randomized
- 4 patients died before receiving their first dose
- 34 patients were excluded for other reasons
- 562 patients included in the analysis
Strengths
- The authors ask a clinically relevant and important question with a patient-centered outcome.
- This was a multicenter trial among 10 different academic centers
- The randomization process was sound and utilized an electronic web-based system.
- Limited exclusion criteria
- Blind adjudication of outcomes limits bias in otherwise open-label study design.
- No patients were lost to follow-up or missing outcomes.
Limitations
- Although this is a multicenter trial, all centers were located in Iran, limiting the study’s generalizability to other locations worldwide.
- This study uses a 2×2 factorial design which could overlook how multiple variables may have contributed to the overall outcome.
- The primary outcome was a composite, and not all components are equal.
- Neither patient nor physicians were blinded. An open-label study design increases bias.
- Unfractionated heparin was used for patients with severe kidney disease, which increases bias and could mask the benefits or harms.
- Investigators used a 12% difference in the primary outcome as the minimally clinically important difference. But they did not discuss how they came to this number.
- Investigators used a 1.8 odds ratio to declare noninferiority in the principal safety outcome as the minimally clinically important difference. But again, they did not discuss how they obtained this number.
- There was no systematic screening of thrombotic events.
- D-dimer value was missing in 66.5% of patients due to availability.
- It’s unclear how many patients were eligible but not assessed for inclusion, increasing the possibility of selection bias.
- Patients in the intermediate dose arm had a higher rate of comorbid conditions and history of smoking.
Discussion
-
Study Design
- A 2×2 factorial design was used to allow the investigators to simultaneously study the effects of intermediate vs. standard-dose anticoagulation and the impact of statin use on the primary outcome. This method allows the investigators to study multiple variables at once.
-
VTE Screening
- Thrombotic events were not systematically screened for. The decision to test patients for VTE was up to the clinician. Although, there was blind adjudication of VTE outcomes. The gold standard test was applied selectively (not to all patients), introducing the potential for bias. In an open-label trial, physicians could selectively screen for VTE more aggressively in the prophylactic dose arm and less aggressively in the intermediate dose arm.
-
Critical differences from recent positive trials
- There was no statistically significant difference in the composite outcome or any of the individual secondary outcomes between the two arms of the trial. However, similar trials have found a benefit of using anticoagulation in non-critically ill patients.
- The HEP-COVID trial and the Multiplatform Trial studied patients with a positive COVID test in less than 72 hours.
- The HEP COVID trial also included patients with a D-dimer 4x above the upper limit of normal.
- Patients were eligible for inclusion in this study if COVID-19 positive within 7 days.
- Additionally, D-dimer levels and other measurements of coagulopathy were not available in a large portion of patients. It is possible that this study failed to capture a cohort of patients who may benefit from higher doses of anticoagulation—those earlier in disease with markedly elevated d-dimer levels.
State of Current Evidence:
-
Critically patients hospitalized with COVID-19
- The current evidence supports standard prophylactic anticoagulation.
-
Hospitalized patients with COVID-19:
- Based on The HEP-COVID [PMID: 34617959] Trial and multiplatform trial [PMID: 34351721], the current evidence supports therapeutic anticoagulation.
-
Formerly hospitalized patients with COVID-19:
- The MICHELLE Trial [PMID: 34921756] reported positive findings. However, several methodological flaws raised concerns, and we would use caution before applying results broadly.
-
Patients with COVID-19 who did not require hospitalization:
- There is no evidence to support any anticoagulation strategy.
Author’s Conclusions: “Among patients with COVID-19 admitted to the ICU, intermediate-dose prophylactic anticoagulation, compared with standard-dose prophylactic anticoagulation, did not result in a significant difference in the primary outcome of a composite of venous or arterial thrombosis, treatment with ECMO, or mortality within 30 days. These results do not support the routine empirical use of intermediate-dose prophylactic anticoagulation in unselected patients with COVID-19 admitted to the ICU.”
Clinical Bottom Line:
Despite its limitations, this study shows no evidence to support the use of intermediate-dose anticoagulation over standard prophylactic dose anticoagulation in patients with COVID-19 in the ICU setting.
Guest Post By:

Jessica DiPeri, MD
PGY-2, Emergency Medicine Resident
Saint Joseph’s University Medical Center, Paterson, New Jersey
Email: jtilzer12@gmail.com

Marco Propersi, DO FAAEM
Assistant Professor, Emergency Medicine
Saint Joseph’s University Medical Center, Paterson, New Jersey
Twitter: @marco_propersi
Steven Hochman, MD FACEP
Associate Professor, Emergency Medicine
Saint Joseph’s University Medical Center, Paterson, New Jersey
Twitter: @hochmast
References:
- Klok, F A et al. “Incidence of thrombotic complications in critically ill ICU patients with COVID-19.” Thrombosis research vol. 191 (2020): 145-147. PMID: 32291094
- Rico-Mesa, Juan Simon et al. “The Role of Anticoagulation in COVID-19-Induced Hypercoagulability.” Current cardiology reports vol. 22,7 53. 17 Jun. 2020, PMID: 32556892
- Spyropoulos AC, Goldin M, Giannis D, et al. Efficacy and Safety of Therapeutic-Dose Heparin vs Standard Prophylactic or Intermediate-Dose Heparins for Thromboprophylaxis in High-risk Hospitalized Patients With COVID-19: The HEP-COVID Randomized Clinical Trial [published correction appears in JAMA Intern Med. 2022 Feb 1;182(2):239]. JAMA Intern Med. 2021;181(12):1612-1620. [PMID: 34617959]
- ATTACC Investigators; ACTIV-4a Investigators; REMAP-CAP Investigators, et al. Therapeutic Anticoagulation with Heparin in Noncritically Ill Patients with Covid-19. N Engl J Med. 2021;385(9):790-802. [PMID: 34351721]
Post-Peer Reviewed By: Anand Swaminathan, MD (Twitter: @EMSwami) and Salim R. Rezaie, MD (Twitter: @srrezaie)
The post The INSPIRATION Trial: Intermediate Dose Anticoagulation in Critically Ill Patients with COVID-19 appeared first on REBEL EM - Emergency Medicine Blog.

