
 Background: The limited availability of rapid tests with sufficient sensitivity has been an issue since the beginning of the pandemic. Therefore, multiple clinical decision scores have been studied to overcome this issue. The addition of laboratory and radiological information can improve predictions scores but run the risks of increased ED length of stay and potential infection risk related to repeated patient contact. What is really needed is a score based on clinical assessment alone with high discrimination.
Background: The limited availability of rapid tests with sufficient sensitivity has been an issue since the beginning of the pandemic. Therefore, multiple clinical decision scores have been studied to overcome this issue. The addition of laboratory and radiological information can improve predictions scores but run the risks of increased ED length of stay and potential infection risk related to repeated patient contact. What is really needed is a score based on clinical assessment alone with high discrimination.
Paper: Goodacre S et al. Derivation and Validation of a Clinical Severity Score for Acutely Ill Adults with Suspected COVID-19: The PRIEST Observational Cohort Study. PLoS One 2021. PMID: 33481930 [Access on READ by QxMD]
Clinical Question: Can the PRIEST score predict adverse outcomes in acutely ill adults with suspected COVID-19 infection?
What They Did:
- Mixed prospective and retrospective observational cohort study in 70 EDs across the UK
- Collected presenting information from 22,445 patients with suspected COVID-19
- Split the cohort into a derivation and validation set:
- Used multivariable analysis to determine variables of the score in the derivation set
- Estimated discriminant performance of the PRIEST score in the validation set
The PRIEST Score (MDCalc):
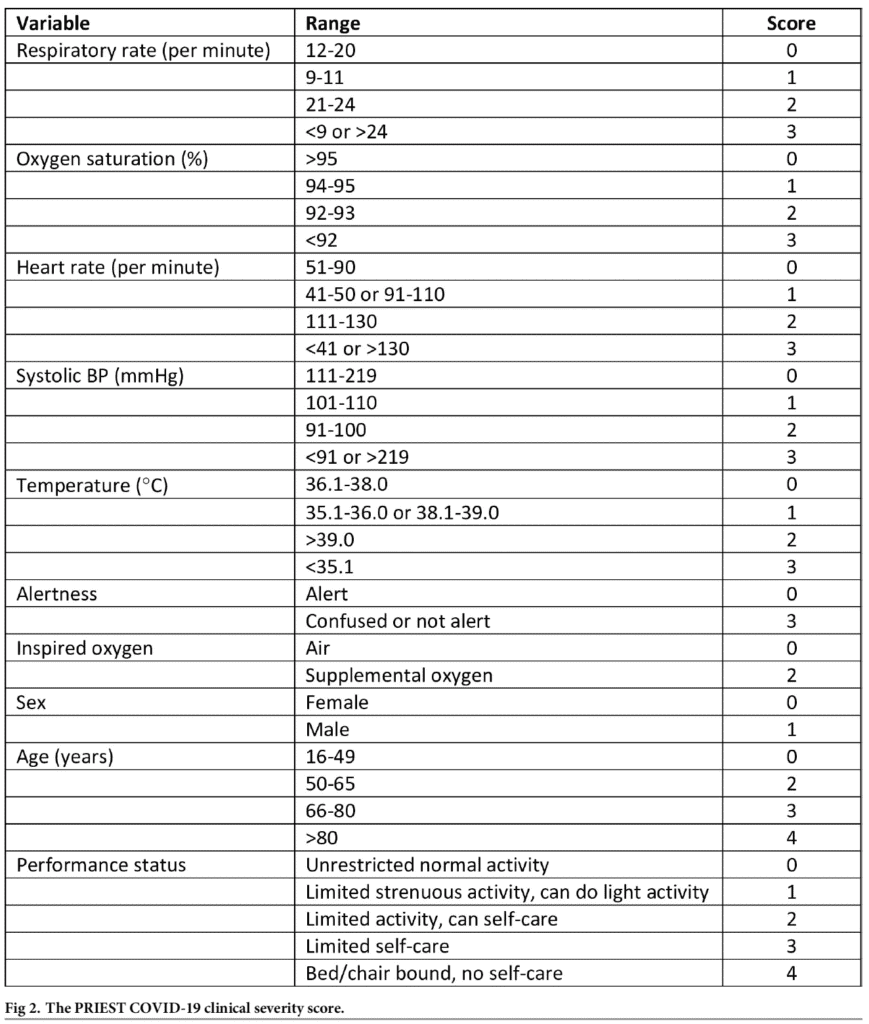
Score ranges from 0 to 29 points (Score >4 predicts adverse outcome with high sensitivity but low specificity)
Outcomes:
-
Primary: Death or organ support (respiratory, cardiovascular, or renal) at 30d
- Identify patients at risk of adverse outcome or requiring life-saving intervention to prevent adverse outcome
- Respiratory: Any intervention to protect the airway or assist ventilation (i.e. NIV, CPAP); DID NOT include supplemental O2 alone or nebulized bronchodilators
- Cardiovascular: Any intervention to maintain organ perfusion (i.e. inotropic drugs or invasive monitoring cardiovascular status with CVP or pulmonary artery pressure monitoring, or arterial blood pressure monitoring); DID NOT include peripheral IV cannulation or fluid administration
- Renal: Any intervention to assist renal function (i.e. hemofiltration, HD, or PD); DID NOT include IV fluid administration
-
Secondary:
- Death
- Major organ support
Inclusion:
- Meeting clinical diagnostic criteria of:
- Fever (≥37.8C)
- Acute onset of persistent cough (with or without sputum)
- Hoarseness
- Nasal discharge or congestion
- Shortness of breath
- Sore throat
- Wheezing
- Sneezing
Exclusion:
- Age or outcome data missing
Results:
- Enrolled Patients:
- Derivation: 11,773 patients
- Validation: 9,118 patients
- Approximately 31% of each cohort had COVID-19 confirmed
- Univariate Analysis Biggest Predictors of Adverse Outcomes:
- Respiratory rate <9: OR 4.72
- Respiratory rate >24: OR 4.62
- Systolic blood pressure <91: OR 4.17
- Temperature <35.1C: OR 3.97
- GCS 9 – 12: OR 5.32
- GCS ≤8: OR 6.49
- Limited Self Care: OR 4.47
- Bed/Chair bound, no self-care: OR 5.45
- Severe respiratory distress: OR 5.46
- Score based on the NEWS2 score, age, sex, and performance status (see PRIEST score above)
- C-statistic = 0.80 (95% CI 0.79 to 0.81)
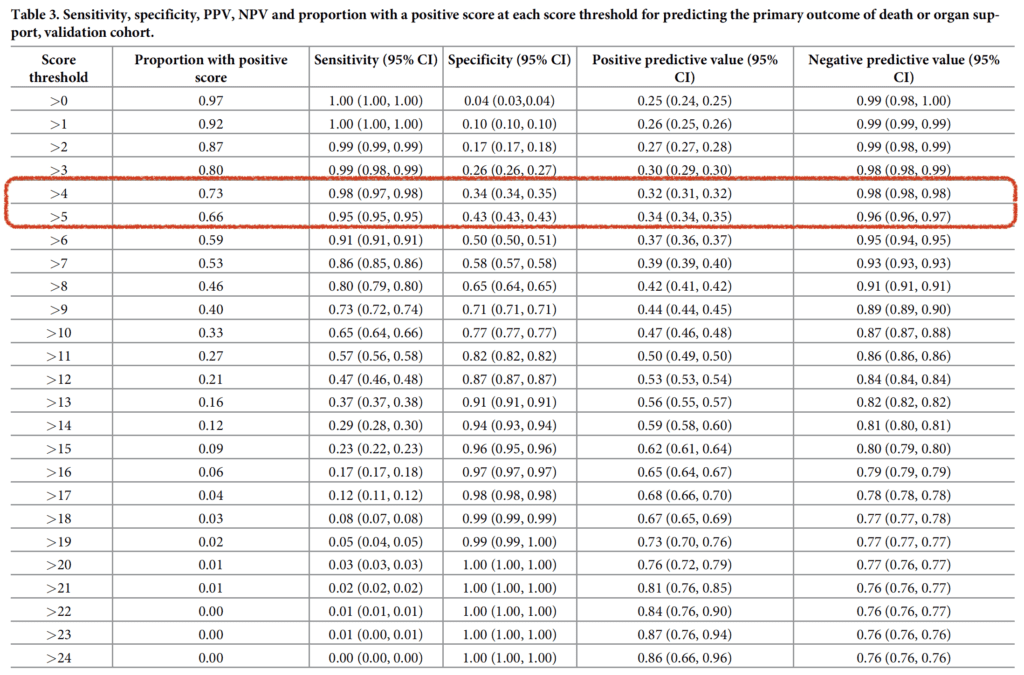
- Score >4 predicted adverse outcome:
- Sensitivity 0.98 (95% CI 0.97 to 0.98)
- Specificity 0.34 (95% CI 0.34 to 0.35)
Strengths:
- Score developed to enhance usability:
- Restricted number of variables
- Very few patients with missing data
- Categorized variables in accordance with currently used scores, unless there was clear evidence that these categories provided suboptimal prediction
- Population of patients randomly separated into derivation and validation cohorts by randomly allocating the participating sites to one or the other cohort
- Used three multivariable analyses using different approaches to missing predictor variable data in the derivation cohort
- Calculated diagnostic parameters at each threshold of the score, constructing a receiver-operating characteristic (ROC) curve, calculating the area under the ROC curve (c-statistic) and calculating the proportion with an adverse outcome at each level of the score
- Compared c-statistic with no limitation of predictors and limited number of predictors and found no major difference in simpler score
Limitations:
- Hospital admission and discharge decisions were made according to usual practice, not based on the PRIEST score
- It’s unclear what the inter-rater reliability was on data extractors
- Outcome at 30 days but is that really what we want? We want to know about early deterioration in the ED
- No comparison to gestalt as most of NEWS2 is inherent in our normal evaluation
- Due to COVID-19 testing only being recommended for admitted patients, it was recorded as a descriptive variable, but not used to select patients or in the analysis
- ≈85% of patients included were Anglo with a lack of minorities represented
- Retrospective data collection resulted in some missing data which could result in some inaccuracy of prediction
- Adverse outcome definition did not include all requirements for hospital admission (i.e. IV fluids and oxygen therapy)
- Any score or triage tool should only support and not replace clinical decision-making
Discussion:
- Authors had created the Pandemic Influenza Triage in the Emergency Department (PAINTED) study following the 2009 H1N1 influenza pandemic to develop and evaluate a triage tool for future influenza pandemics. They changed PAINTED to the Pandemic Respiratory Infection Emergency System Triage (PRIEST) study in January 2020 to address any pandemic respiratory infection, including COVID-19
- The PRIEST SCORE:
- Rigorous derivation and validation
- Independently peer-reviewed protocol set up in advance of the pandemic
- Large and representative cohort presenting to EDs
- Analyzed using a pre-specified statistical analysis plan
- The authors put up a nice table to show the sensitivity, specificity, PPV, and NPV at varying cutoffs of the PRIEST score. At my institution we are currently using a cutoff of 5 as opposed to the 4 that was suggested in this study:
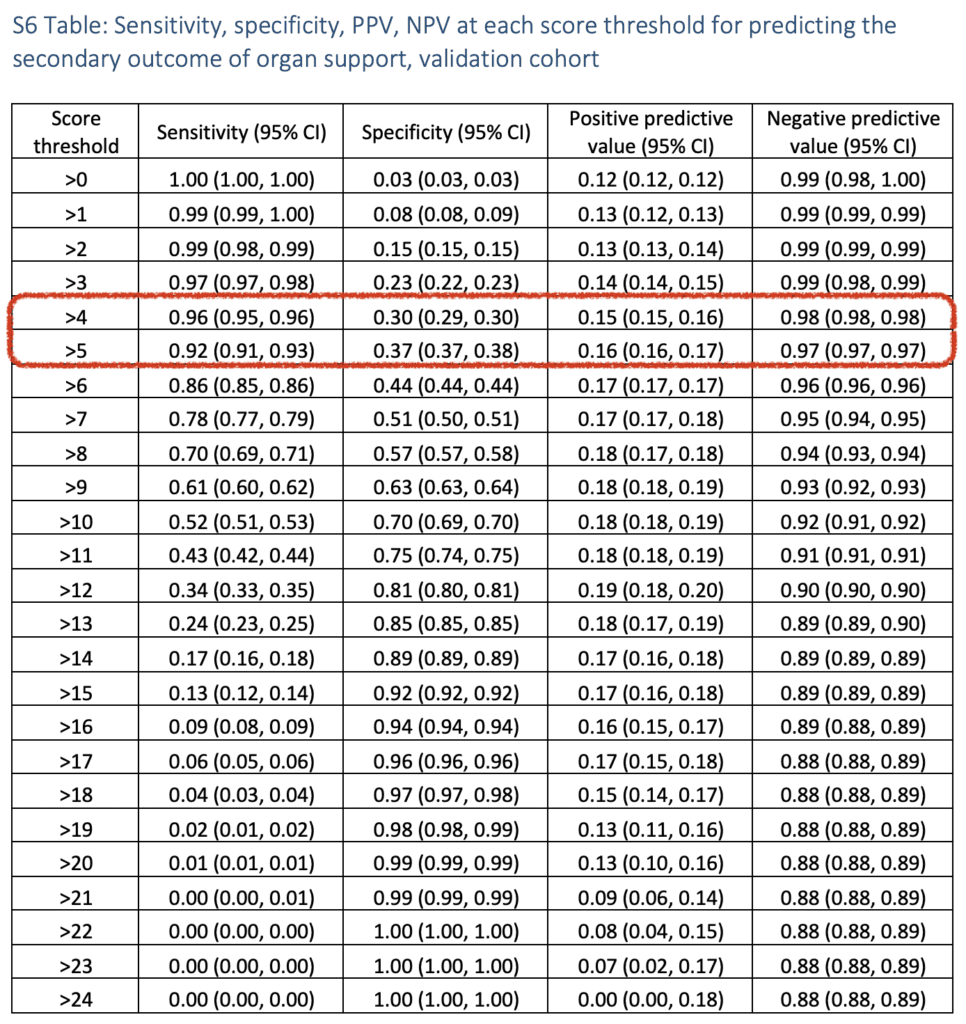
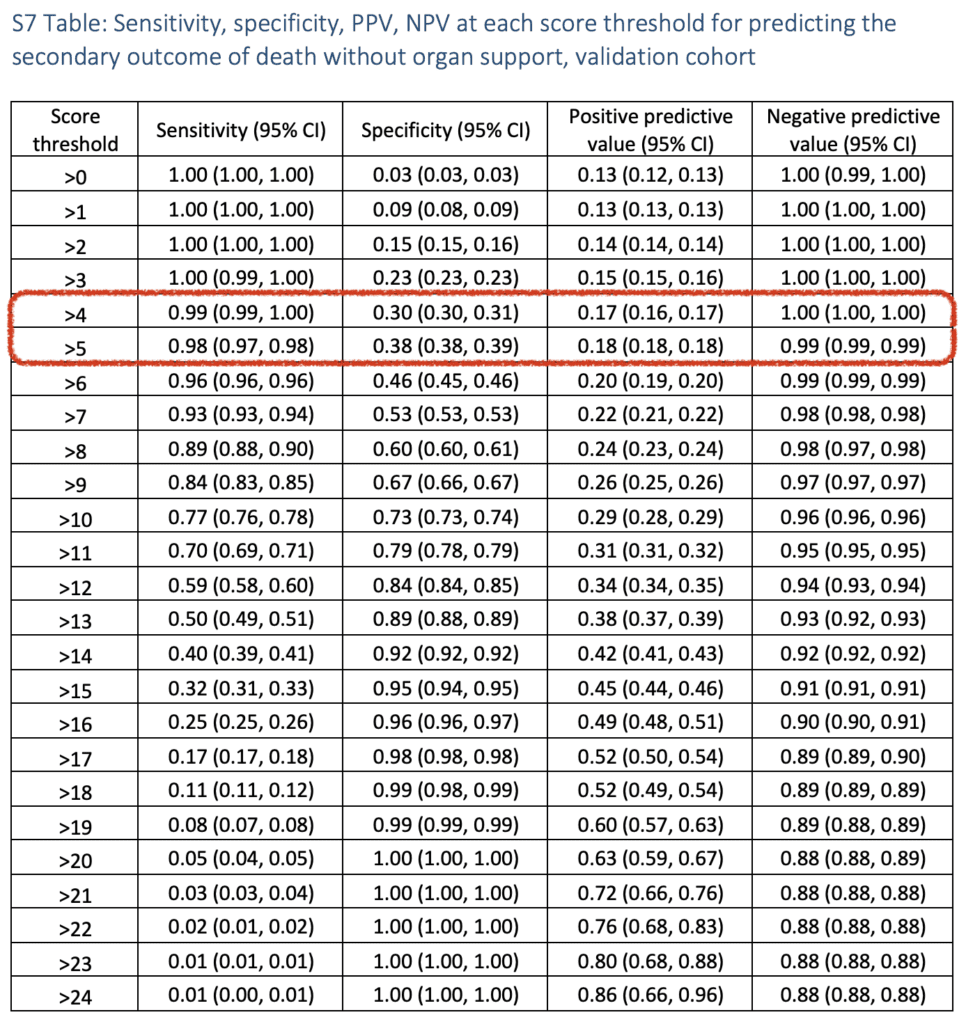
- This was also done for the secondary outcomes of predicting organ support alone and mortality:
- C-statistic for existing scores used to evaluated acute COVID-19 discussed in the study:
- CURB-65 0.75
- NEWS2 0.77
- PMEWS 0.77
- Limitations of other risk stratification scores:
- Only predict mortality
- Most developed on admitted populations
- Inclusion of laboratory/radiographical variables prolongs ED length of stay and prevents rapid assessment
- Limited by small numbers of patients producing imprecise estimates of accuracy
- Single center design limiting generalizability
Author Conclusion: “A clinical score based on NEWS2, age sex, and performance status predicts adverse outcome with good discrimination in adults with suspected COVID-19 and can be used to support decision-making in emergency care.”
Clinical Take Home Point: The PRIEST score is a rapid assessment tool that can aid in the prediction of adverse outcomes in adults who are acutely ill with suspected COVID-19 presenting to the ED at 30 days. This is promising, however a score that can predict 48-to-72-hour outcomes would be more meaningful.
References:
- Goodacre S et al. Derivation and Validation of a Clinical Severity Score for Acutely Ill Adults with Suspected COVID-19: The PRIEST Observational Cohort Study. PLoS One 2021. PMID: 33481930 [Access on READ by QxMD]
Post Peer Reviewed By: Anand Swaminathan, MD (Twitter: @EMSwami)
The post The PRIEST Score: Predicting Adverse Outcomes in COVID-19 appeared first on REBEL EM - Emergency Medicine Blog.
