 Background: The scientific process in medicine is complicated. Obtaining high-quality data to guide management requires hypothesis formulation, data to support the hypothesis and study replication. Time and again beneficial findings in therapeutic studies fail to be replicated in subsequent studies. A single positive trial may cause some to feel it unethical to assign patients to a standard therapy that could potentially deprive them of benefit. Alternatively, pharmaceutical companies have little impetus to attempt or support collecting additional data that may jeopardize their product. In research, repetition is the pillar on which clinical trials results should be founded on. As this may not be feasible, complete transparency of all aspects of a trial are essential.
Background: The scientific process in medicine is complicated. Obtaining high-quality data to guide management requires hypothesis formulation, data to support the hypothesis and study replication. Time and again beneficial findings in therapeutic studies fail to be replicated in subsequent studies. A single positive trial may cause some to feel it unethical to assign patients to a standard therapy that could potentially deprive them of benefit. Alternatively, pharmaceutical companies have little impetus to attempt or support collecting additional data that may jeopardize their product. In research, repetition is the pillar on which clinical trials results should be founded on. As this may not be feasible, complete transparency of all aspects of a trial are essential.
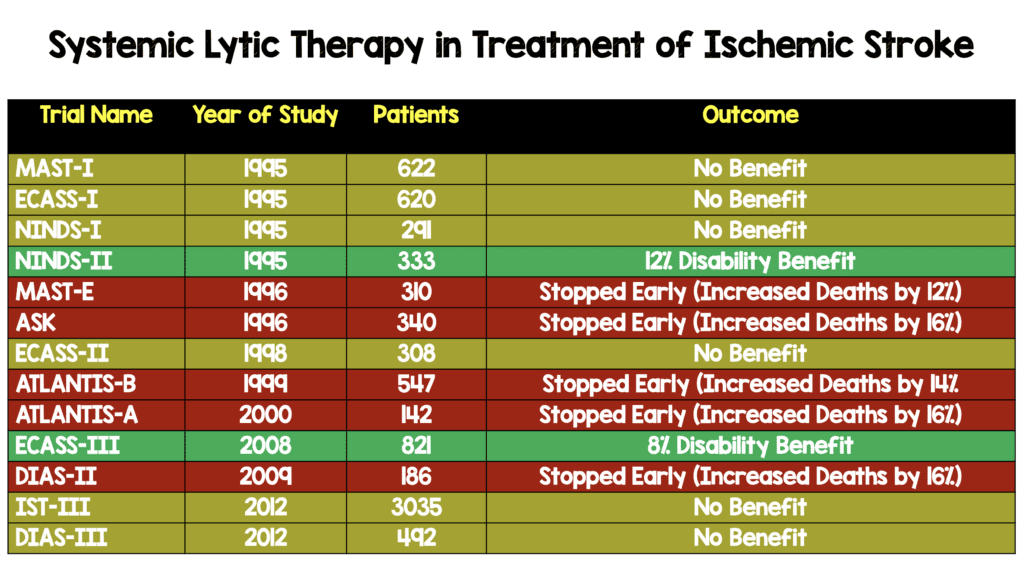
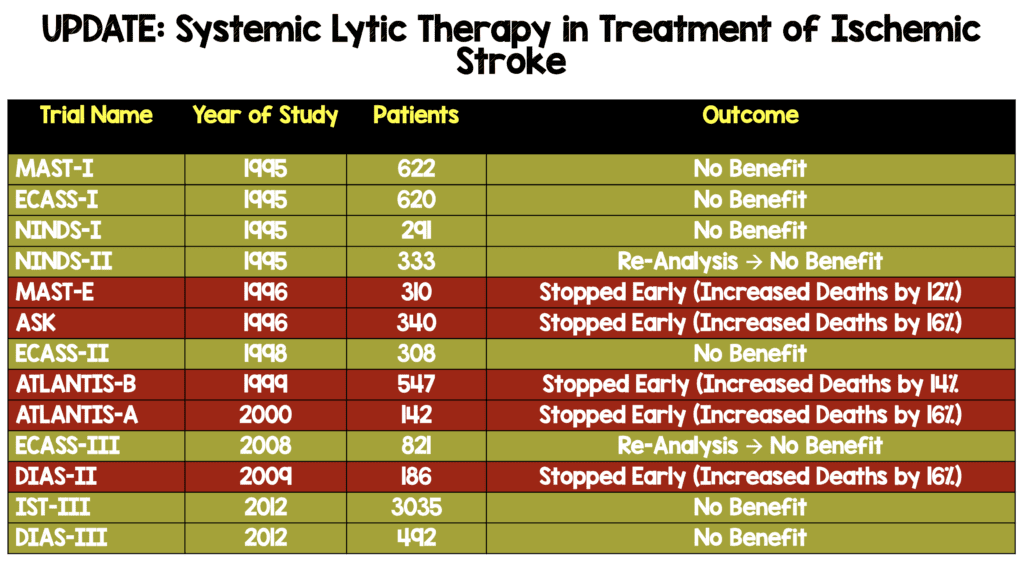
One of the most hotly debated topics in emergency medicine is the use of systemic thrombolysis in acute ischemic stroke. There are only two randomized clinical trials that demonstrate benefit in neurologic outcomes: NINDS-II and ECASS-III (see table below). Methodological experts, however, have raised concerns that both studies had baseline imbalances in stroke severity that may have biased the trials final results. Both studies have undergone re-analysis taking these baseline differences into account.
Systemic Lytic Therapy in Treatment of Ischemic Stroke Prior to Re-Analysis
Paper: Hoffman J et al. A Graphic Reanalysis of the NINDS Trial. Ann Emerg Med 2009. PMID: 19464756
Clinical Question: Does removing confounders and effect modifiers in patients from the original NINDS trials change the outcomes of alteplase given within 3 hours of symptom onset for acute ischemic stroke?
What They Did:
- Used the original data from the two NINDS trials to create graphs showing the effect of treatment on 90-day neurologic function stratified by relevant confounders and effect modifiers
- Analysis restricted to time to treatment, age, disability before the event, and baseline NIHSS score
Outcomes:
- Neurologic improvement stratified to potential confounders and effect modifiers:
- Time to treatment
- Age
- Disability before the event
- Baseline NIHSS score
Inclusion/Exclusion:
- Same as original NINDS trials
Results:
- 624 patients
- Cumulative distribution of 90d NIHSS for patients grouped by treatment (placebo vs tPA) and time treatment was given (0 to 90min vs 90 to 180min)
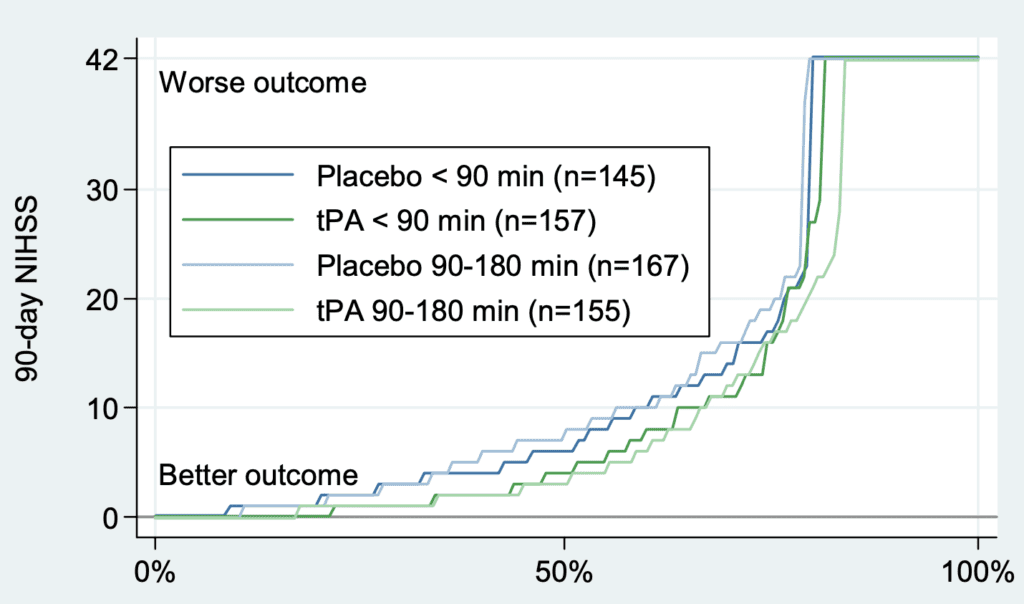
- All curves are similar but those of tPA are slightly better than those of placebo, suggesting that tPA may offer a small benefit. However, when cumulative change in NIHSS score from baseline to 90 days are evaluated, much of the difference between tPA and placebo disappears, suggesting confounders in the above graph
- Distribution of baseline, 90d, and change in NIHSS by treatment
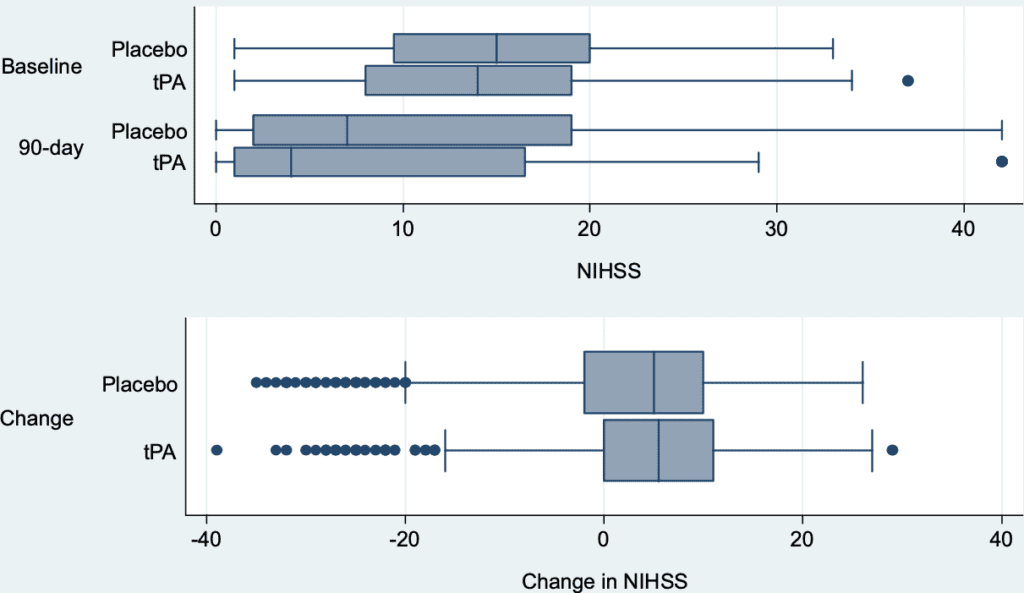
- At baseline, patients in the placebo group where more neurologically impaired compared to tPA group (top panel). However, distributions of 90d change in NIHSS (bottom panel) are similar
- No effect of time to treatment on 90d neurologic outcomes with tPA compared to placebo
- Half of the patients were treated within 90 minutes of stroke onset and the other half between 90 and 180 minutes
- Re-analyzing data for only patients who remained alive at 90d, there was also no improvement in functional outcome in survivors with tPA compared to placebo
Strengths:
- Used multiple formats and scales to reflect the full range of values of outcomes from individual patient level data
- Used 2 methods to control for potential confounding by imbalances in initial stroke severity between groups
Limitations:
- Original data set only recorded one blood pressure measurement per patient which did not allow for assessment of this potential confounder
- Analysis limited by the constraints of the original trial
- NIHSS scale is ordinal (ordered categories but the distances between categories is not known): A change from NIHSS of 10 to 7 is not equivalent to a change from 15 to 12
Discussion:
- The fragility index of the NINDS trial = 3 (i.e. only 3 patients would have to have a different outcome to change the study from positive to negative)
- Baseline stroke severity and preexisting disability have the largest association with outcome than age or treatment provided (i.e. Final outcomes were highly dependent on stroke severity)
- This re-analysis shows that most patients who survive improve in neurologic status, regardless of treatment. This is not the case for patients with a baseline NIHSS value between roughly 5 and 22, where there is a trend toward better outcomes for tPA compared to placebo. The reverse was true for patients with mild or more severe initial stroke scores.
- The big outcome that was highlighted in NINDS is the mRS (not change in mRS but rather percent that are 0-1). If the patients are starting with higher mRS (i.e. higher disability), less likely to get to target endpoint (i.e. placebo).
- Although there was a very small difference in NIHSS improvement with tPA vs placebo on this re-analysis, there are some important things to consider:
- The trial is small enough that observed differences may be random statistical noise
- The trial is small enough that randomization failed to produce comparable groups
Author Conclusion: “Our graphical method of presenting the NINDS trial results provides more detail than was conveyed in the original report and empowers readers to reach their own conclusions about the trial’s meaning. Outcomes for placebo and treatment limbs are sufficiently similar that larger trials, conducted under the same conditions as the NINDS trial, are needed to determine which patients benefit from this therapy.”
Clinical Take Home Point: This re-analysis does not answer the question of whether tPA within 3 hours from symptom onset is beneficial compared to placebo in patients with acute ischemic stroke but does support the need for further large RCTs as this data raises more questions than answers about tPA.
Paper: Alper BS et al. Thrombolysis With Alteplase 3 – 4.5 Hours After Acute Ischaemic Stroke: Trial Reanalysis Adjusted for Baseline Imbalances. PMID: 32430395
Clinical Question: Does adjusting for baseline imbalances from the original ECASS-III trial change the outcomes of alteplase given within 3 to 4.5 hours from symptom onset for acute ischemic stroke?
What They Did:
- Re-analysis of ECASS III trial data adjusting for baseline imbalances to determine robustness or sensitivity of the efficacy estimates of tPA vs placebo
Outcomes:
- Primary: Adjusted reanalysis of:
- Symptom free status (mRS 0) at 90d
- Dependence-free status (mRS 0 to 1) at 90d
- Dependence-free status (mRS 0 to 2) at 90d
- Mortality (mRS 6) at 7d
- Change across mRS 0 to 6 spectrum at 90d
- Mortality at 90d
- Symptomatic ICH at 7 days
Inclusion/Exclusion:
- Same as the original ECASS-III trial
Results:
- 821 adults
- Only two variables were statistically significant: Baseline imbalances in NIHSS score and history of prior stroke. After balancing for these two confounders:
- No statistical difference in treatment effect of tPA vs placebo in any of the outcomes
- There was an increase in symptomatic ICH with alteplase vs placebo
Strengths:
- Applied 3 analytical approaches including multivariable modeling, matching, and stratified analysis to adjust for potential confounders
- Prespecified outcomes of clinical importance to avoid selective reporting
- Prespecified analytical methods to avoid selective analysis
Limitations:
- Reanalysis is limited to the quality of the trial data from ECASS III
- Reanalysis cannot produce new conclusions but can only increase or decrease the certainty in the unadjusted analysis
Discussion:
- The fragility index of the original ECASS III trial is 1 (Only one patient would have to have a different outcome to change the study form positive to negative)
- In the original ECASS III trial, baseline NIHSS score was lower in the alteplase group (mean 10.7) vs placebo (11.6) and more previous stroke in the placebo group (14.1%) vs tPA group (7.7%). Both of these differences bias the results in favor of alteplase
Author Conclusion: “Reanalysis of the ECASS III trial data with multiple approaches adjusting for baseline imbalances does not support any significant benefits and continues to support harms for the use of alteplase 3—4.5 hours after stroke onset. Clinicians, patients and policymakers should reconsider interpretations and decisions regarding management of acute ischaemic stoke that were based on ECASS III results.”
Clinical Take Home Point: The re-analysis of ECASS III raises concerns that baseline imbalances create bias and affect the conclusions of efficacy of alteplase given within 3 to 4.5 hours after stroke onset in acute ischemic stroke compared to placebo.
Clinical Bottom Line: There have been only two randomized clinical trials to show benefit of systemic alteplase vs placebo in acute ischemic stroke. After balancing for baseline differences in patients between the two groups, re-analysis of these two trial questions the validity and robustness of alteplase. This means there are now ZERO RCTs supporting the practice of thrombolysis in acute ischemic stroke.
Systemic Lytic Therapy in Treatment of Ischemic Stroke After Re-Analysis
References:
- Hoffman J et al. A Graphic Reanalysis of the NINDS Trial. Ann Emerg Med 2009. PMID: 19464756
- Alper BS et al. Thrombolysis With Alteplase 3 – 4.5 Hours After Acute Ischaemic Stroke: Trial Reanalysis Adjusted for Baseline Imbalances. PMID: 32430395
For More Thoughts on This Topic Checkout:
- Lown Institute: Will it Take 50 – 100 Years to Get the Right Answer About tPA for Stroke?
- The SGEM: SGEM #297 – tPA Advocates Be Like – Never Gonna Give You Up
Post Peer Reviewed By: Anand Swaminathan, MD (Twitter: @EMSwami)
The post Thrombolysis in Acute Ischemic Stroke: Now We Have No Positive RCTs appeared first on REBEL EM - Emergency Medicine Blog.