
 Recently, there has been a lot of buzz about the use of topical tranexamic acid for epistaxis or oral bleeds on multiple social media platforms. Everyone seems so happy that it works so well, but we thought we would look through the literature and see what the evidence for use of topical tranexamic acid (TXA) is and how best to compound it for these clinical dilemma. We performed a PubMed, and Ovid search using the terms “topical” AND/OR “oral solution” AND/OR “intranasal” PLUS “tranexamic acid” to answer our questions at hand.
Recently, there has been a lot of buzz about the use of topical tranexamic acid for epistaxis or oral bleeds on multiple social media platforms. Everyone seems so happy that it works so well, but we thought we would look through the literature and see what the evidence for use of topical tranexamic acid (TXA) is and how best to compound it for these clinical dilemma. We performed a PubMed, and Ovid search using the terms “topical” AND/OR “oral solution” AND/OR “intranasal” PLUS “tranexamic acid” to answer our questions at hand.
Clinical Questions:
- What is the literature regarding the use of topical tranexamic acid for epistaxis or oral bleeds?
- How should it be compounded and administered safely and effectively?
Clinical Answer:
While there is little evidence available directly regarding the use of topical tranexamic acid (TXA) for epistaxis or oral bleeds in the emergency department (ED), the use of topical TXA has been used for epistaxis, hyphema, and dental extractions in a variety of settings. Its use has been studied in patients both on and off oral anticoagulants and with or without bleeding disorders such as hemophilia. It can be extrapolated from the studies that topical TXA is effective in stopping or controlling bleeds, and has been shown to be safe for use in these cases. Use of topical TXA in epistaxis has also been shown to reduce time spent in the ED. Topical solutions can be compounded as a 5% solution using the intravenous solution (100mg/mL) or a 3.25% solution using a 650 mg oral tablet dissolved in water and stored for up to 5 days in the refrigerator.
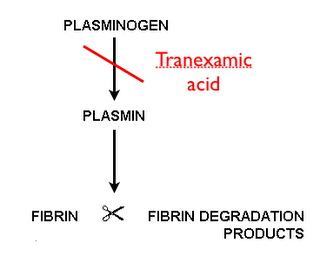
Review of the Evidence for Topical Tranexamic Acid for Epistaxis or Oral Bleeds
Background:
TXA is an antifibrinolytic drug which reversibly binds to plasminogen and prevents its interaction with fibrin, thus inhibiting the dissolution of fibrin clots. TXA is available in both a 100 mg/mL solution for injection, and an oral 650 mg tablet. The intravenous (IV) solution is FDA approved in patients with hemophilia for short-term use (two to eight days) to reduce or prevent hemorrhage and reduce the need for replacement therapy during and following tooth extraction while the oral (PO) formulation is FDA approval for the treatment of cyclic heavy menstrual bleeding. Despite the narrow indications for which TXA is approved in the United States, it has been studied in a wide variety of conditions in which systemic or local hyperfibrinolysis is involved including cardiac surgery, orthopedic surgery, spinal surgery, post-partum hemorrhage, gastrointestinal bleeding, trauma, epistaxis, hyphema, and dental extractions [1]. The formulation of TXA has not been standardized across the various studies with a range of IV, PO, and topical solutions being used. Topical TXA is an attractive option as it would inhibit local fibrinolysis at the site of bleeding with minimal systemic absorption [2]. This review will focus on the topical use of TXA in localized hyperfibrinolysis such as epistaxis, dental procedures, and hyphema.
TXA in epistaxis:
In a randomized controlled trial conducted in an emergency department, 216 patients presenting with ongoing epistaxis and known history of bleeding disorders (thrombocytopenia, hemophilia, platelet disorders) were randomized to receive either packing soaked in TXA (500 mg in 5 mL) or packing soaked in epinephrine plus lidocaine followed by packing covered in tetracycline for three days (anterior nasal packing) [3]. At baseline, patients who received TXA as treatment were more likely to have a history of epistaxis (p=0.001), but all other baseline characteristics such as age, sex, platelets, PT/INR were similar. There were statistically significant differences in bleeding stop time <10 min (71% vs 31.2%; OR 2.28, p<0.001) and discharge from the ED in <2 hours (95.3% vs 6.4%;OR 14.8, p<0.001). There was also a higher rate of rebleeding in the first 24 hours and within 1 week in the group treated with anterior nasal packing as compared to those treated with TXA. There was no statistical significance between complications in the ED. Limitations to be noted are that posterior epistaxis was not included, and patients with a baseline INR >1.5, shock, and visible bleeding vessels were excluded.
TXA in hyphema:
Hosseini et al. conducted a comparative study of 30 patients with traumatic gross hyphema with either TXA 5% eye drop (TXA 500 mg/5 mL in 5 mL artificial tear eye drop containing hydroxypropyl methyl, cellulose, and dextran) every 6 hours for 5 days as compared to a historical group of patients with hyphema receiving oral placebo or oral TXA [4]. Exclusion criteria were microscopic hyphema, ruptured globe, posterior segment injuries other than commotio retina, diabetes mellitus, hypertension, coagulative disorders, receipt of anticoagulants, pregnant and nursing women, and children <7 years old. Rate of rebleeding were evaluated at day 4, 8, and 14. Only one patient (3.3%) experienced rebleeding as compared to 26% in the historical cohort receiving oral placebo (p=0.008), and 10% of patients receiving oral TXA (p=0.25). In both cases, the cohort receiving topical TXA was significantly older, and had a significantly lower intraocular pressure at baseline. Treatment with topical TXA was well tolerated, and the authors concluded that topical TXA may be an option for treatment of hyphema grades 1-3, although the study design and small sample size limited them from making any decisive conclusions. The strict exclusion criterion limits the use of this study in a broad patient population.
Dental:
The largest body of evidence for use of topical TXA exists in the dental literature. The evidence applies to both peri-operative and post-operative uses, with varying results. Use of 10 mL of 0.05% TXA (500 mg TXA in 1 L normal saline) versus 10 mL normal saline irrigating fluid during bimaxillary osteotomy was found to have no difference on mean intraoperative blood loss, first and third postoperative day hematocrit levels, blood transfusion requirement and amount of irrigant fluid in 40 patients [5]. Anecdotally, one treatment site in South Africa has used crushed TXA tablets 500 mg suspended in water for dental surgeries and tooth extractions post-operatively [6]. Patients with inherited bleeding disorders would either swish and swallow the solution, or bite down for thirty minutes on cotton soaked in the TXA solution with reported success in “most cases.”In a double-blind randomized clinical trial studying the use of TXA solution in patients with inherited bleeding disorders receiving dental scaling, patients were instructed to dissolve 500 mg TXA tablets in 10 mL distilled water, then swish for two minutes followed by expectoration [7]. As compared to treatment with IV factor transfusion followed by a placebo mouthwash (performed on the same cohort with two weeks separation), topical TXA was found to be non-inferior and an effective alternative to factor therapy in controlling gingival hemorrhage.
Use in patients taking Anticoagulants and dental procedures:
There are several studies from older literature showing the safety of topical TXA use in patients currently on warfarin. In two separate studies performed over a decade ago, use of a 4.8-5% TXA mouth rinse from two to seven days was shown to be effective (regardless of treatment duration) in patients with dental extractions and no withdrawal of oral anticoagulants [8][9]. Systemic treatment could possibly lead to the development of thromboembolism, especially this high risk population, but oral use of 10 mL of a 4.8% TXA solution available in Australia led to an average concentration of only 7 μg of TXA acid/mL saliva after two hours when used as swish and spit [10]. The plasma concentration was found to be barely detectable after use of the mouthwash, supporting the conclusion that there is insignificant inhibition of systemic fibrinolysis and side effects would not be expected.
Safety
In a Cochrane review of the topical use of TXA in intra-cardiac surgery, orthopedic surgery, epistaxis, and dental extractions, there were no adverse events reported regarding myocardial infarction, pulmonary embolism, stroke, or deep vein thrombosis. It was reported to incidentally reduce the need for blood transfusions by 45% [2]. Based on these results and results of studies included in this analysis including a wide variety of patients, topical TXA may be considered safe for use.

Compounding
The studies cited above used a variety of methods for extemporaneous compounding of TXA ranging from 0.05% to 10% solutions. Two methods have been suggested depending on the formulation used [11]. If the 100 mg/mL or 10% solution for injection is used, a 5% oral solution can be prepared by diluting 5 mL of tranexamic acid with 5 mL of sterile water. If the 500 mg tablets are available, one tablet can be placed into 20 mL of water, and stirred until the tablets are completely disintegrated to form a fine particular suspension. It is suggested that a maximum expiration date of five days should be employed if refrigerated, and the solution should be protected from light.3 In the place of a 500 mg tablet, it seems that use of a 650 mg tablet dissolved in 20 mL could be safely used as well. This is supported by evidence in which a 500 mg tablet was dissolved in 10 mL of water with no reports of adverse events [7]. There is evidence to support that an even lower concentration may be effective, as a 0.05% solution was utilized by Kaewpradub and colleagues, although higher concentrations have safely been used.
Guest Post by:
 Courtney Waye, PharmD
Courtney Waye, PharmD
PGY2 Internal Medicine Pharmacy Resident
South Texas Veterans Health Care System
References:
- McCormack PL. Tranexamic Acid: A Review of its use in the Treatment of Hyperfibrinolysis. Drugs 2012. PMID: 22397329
- Ker K et al. Topical Application of Tranexamic Acid for the Reduction of Bleeding. Cochrane Database Syst Rev 2013. PMID: 23881695
- Zahed R et al. A New and Rapid Method for Epistaxis Treatment Using Injectable arm of Tranexamic Acid Topically: A Randomized Controlled Trial Am J Emerg Med 2013. PMID: 23911102
- Jahadi H et al. Comparison Between Topical and Oral Tranexamic Acid in Management of Traumatic Hyphema. Iran J Sci Med 2014. PMID: 24753640
- Kaewpradub P et al. Does Tranexamic Acid in an Irrigating Fluid Reduce Intraoperative Blood Loss in Orthognathic Surgery? A Double-Blind, Randomized Clinical Trial J Oral Max Surg 2011. PMID: 21398011
- Coetzee MJ et al. The use of Topical Crushed Tranexamic Acid Tablets to Control Bleeding After Dental Surgery and from Skin Ulcers in Hemophilia. Haemophilia 2007. PMID: 17610565
- Nuvvula S et al. Efficacy of Tranexamic Acid Mouthwash as an Alternative for Factor Replacement in Ginigival bleeding During Dental Scaling in Cases of Hemophilia: A Randomized clinical Trial. Contemp Clin Dent 2014. PMID: 24808695
- Carter G et al. Tranexamic Acid Mouthwash: A Prospective Randomized Study of a 2-Day Regimen vs 5-Day Regimen to Prevent Postoperative Bleeding in Anticoagulated Patients Requiring Dental Extractions. Int J Oral Max Surg 2003. PMID: 14759109
- Borea G et al. Tranexamic Acid as a Mouthwash in Anticoagulant-Treated Patients undergoing Oral Surgery. an alternative Method to Discontinuing Anticoagulant Therapy. Oral Surg Oral Med Oral Path 1993. PMID: 8419869
- Carter G et al. Current concepts of the Management of Dental Extractions for patients Taking Warfarin. Aust Dent J 2003. PMID: 14649397
- Lam MS et al. Extemporaneous Compounding of Oral Liquid Dosage Formulations and Alternative Drug Delivery methods for Anticancer Drugs. Pharmacotherapy 2011. PMID: 21275495
For more on this topic checkout:
- Ken Milne over at The Skeptics Guide to Emergency Medicine (The SGEM): SGEM #53 – Sunday, Bloody Sunday (Epistaxis and Tranexamic Acid)
The post Topical Tranexamic Acid for Epistaxis or Oral Bleeds appeared first on REBEL EM - Emergency Medicine Blog.
