 The Novel Coronavirus 2019, was first reported on in Wuhan, China in late December 2019. The outbreak was declared a public health emergency of international concern in January 2020 and on March 11th, 2020, the outbreak was declared a global pandemic. The spread of this virus is now global with lots of media attention. The virus has been named SARS-CoV-2 and the disease it causes has become known as coronavirus disease 2019 (COVID-19). This new outbreak has been producing lots of hysteria and false truths being spread, however the data surrounding the biology, epidemiology, and clinical characteristics are growing daily, making this a moving target. This post will serve as a summary of a clinical/therapeutic staging proposal and treatment in regard to COVID-19.
The Novel Coronavirus 2019, was first reported on in Wuhan, China in late December 2019. The outbreak was declared a public health emergency of international concern in January 2020 and on March 11th, 2020, the outbreak was declared a global pandemic. The spread of this virus is now global with lots of media attention. The virus has been named SARS-CoV-2 and the disease it causes has become known as coronavirus disease 2019 (COVID-19). This new outbreak has been producing lots of hysteria and false truths being spread, however the data surrounding the biology, epidemiology, and clinical characteristics are growing daily, making this a moving target. This post will serve as a summary of a clinical/therapeutic staging proposal and treatment in regard to COVID-19.
To go back to the main post, click on the image below…
Clinical-Therapeutic Staging Proposal [8]
Staged progression of COVID-19 illness can help in deployment and investigation of target therapy
- In this editorial the authors propose a clinical staging system to establish a standardized nomenclature for evaluation and reporting of disease
- Pharmacotherapy targeted against the virus holds the greatest promise when applied early in the course of disease, but its usefulness in advanced stages may be futile
- Anti-inflammatory therapy that is applied too early may not be necessary as most people will only have mild disease and recover without any difficulty. This may even prolong viral replication which has been described with corticosteroids.
- Proposed Clinical Staging System:
- Stage I: Mild (Early Infection)
- Stage IIa: Moderate (Pulmonary Involvement Without Hypoxia)
- Stage IIb: Moderate (Pulmonary Involvement With Hypoxia)
- Stage III: Severe (Systemic Hyperinflammation)
Stage I Mild (Early Infection):
- This occurs at time of inoculation, incubation, and early establishment of disease
- This is typically non-specific symptoms (i.e. malaise, cough, fever, etc.)
- Depending on where you work, diagnosis is made by respiratory RT-PCR testing, serum testing from SARS-CoV-2 IgG and IgM, chest imaging, CBC (i.e. lymphopenia, leukopenia, etc.), and LFTs
- Treatment should focus on symptomatic relief
- Should anti-viral therapy become available, this group of patients may have benefit in reducing duration of symptoms, minimizing contagiousness, and potentially progression of disease
Stage II – Moderate (Pulmonary Involvement with and without Hypoxia)
- This occurs with establishment of pulmonary disease and inflammation in the lung
- Patients can develop viral pneumonia, with cough, fever, and maybe hypoxia (Defined as a PaO2/FiO2 of <300mmHg)
- Imaging (CXR and/or Chest CT) can show bilateral infiltrates or ground glass opacities
- CBC can have increasing lymphopenia and transaminitis
- Procalcitonin is typically low to normal in most cases of COVID-19 PNA
- These patients will most likely need to be hospitalized for observation and management
- Treatment will again focus on symptomatic measures
- In Stage IIa (Without Hypoxia) patients, corticosteroids should be avoided
- In Stage IIb (With Hypoxia) patients, and patients require mechanical ventilation, anti-inflammatory therapy could be useful but used judiciously
- Some places may have the ability to use anti-viral therapy under “compassionate” measures
Stage III – Severe (Systemic Hyperinflammation):
- This will be a minority of COVID-19 patients
- This occurs when extra-pulmonary systemic hyperinflammation occurs (i.e. cytokine storm)
- Can see elevations in IL-2, IL-6, IL-7, granulocyte-colony stimulating factor (GCSF), macrophage inflammatory protein 1-alpha, TNF alpha, CRP, ferritin, and D-dimer
- Can also see elevations in troponin and BNP
- In this stage, tailored therapy will hinge on use of immunomodulatory agents to reduce systemic inflammation
- Corticosteroids may be justified in conjunction with cytokine inhibitors such as tocilizumab (IL-6 inhibitor) or anakinra (IL-1 receptor antagonist)
- IVIG may also play a role
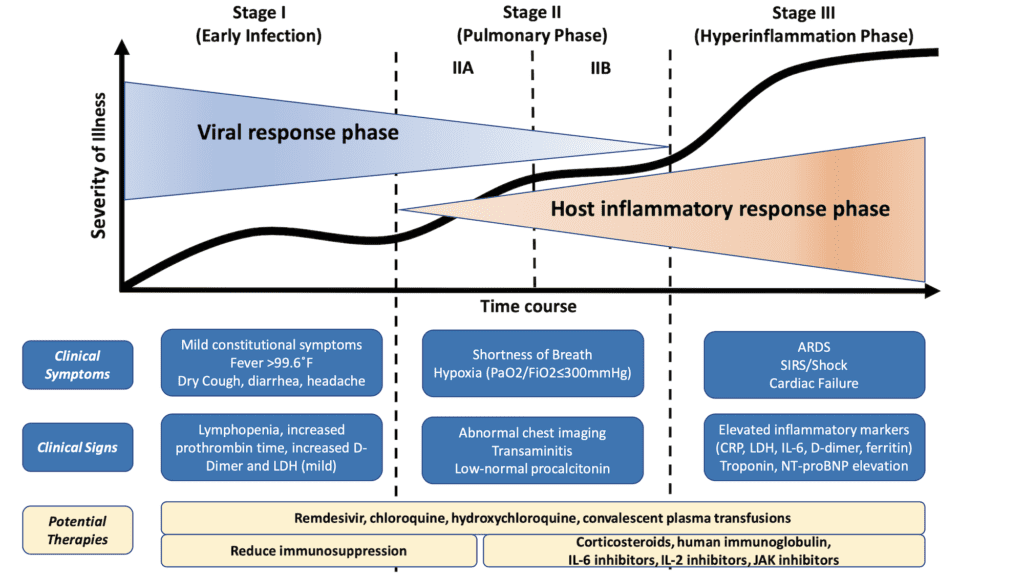
Figure 1: Classification of COVID-19 Disease States and Potential Therapeutic Targets [8][Link is HERE]
Hypothetical Pathogenesis of COVID-19 [26]
- Immune mediated inflammation plays an important role in the pathogenesis
- High proportion of aberrant coagulation in severe and critical patients with COVID-19, although rare in other coronavirus infections, has been reported in severe influenza
- clinical course of SARS-CoV-2 infection could be divided into 3 phases:
- Viremia phase
- Acute phase (Pneumonia phase)
- Severe or recovery phase
- Patients with competent immune function and without obvious risk factors (i.e. old age, comorbidities, etc.) can generate effective and adequate immune responses to suppress the virus in the 1st or 2nd phase without immune over-reaction
- Patients with immune dysfunction may have a higher risk of failing virus suppression and hence become more severe or critical with higher mortality
- Treatment should be targeted at the risk factors of patients and the window of opportunity may lie between the 1st and 2nd phases when clinical deterioration is asserted with evidence of abrupt inflammation and hypercoagulable status
- There is no consensus regarding the best management options, but potential measures include:
- Glucocorticoids
- High-dose IVIG
- Anti-IL-6R Antibody (i.e. Tocilizumab, etc.)
- Convalescent plasma therapy
- Take home message: Timing is extremely important, regardless of what treatment might be used
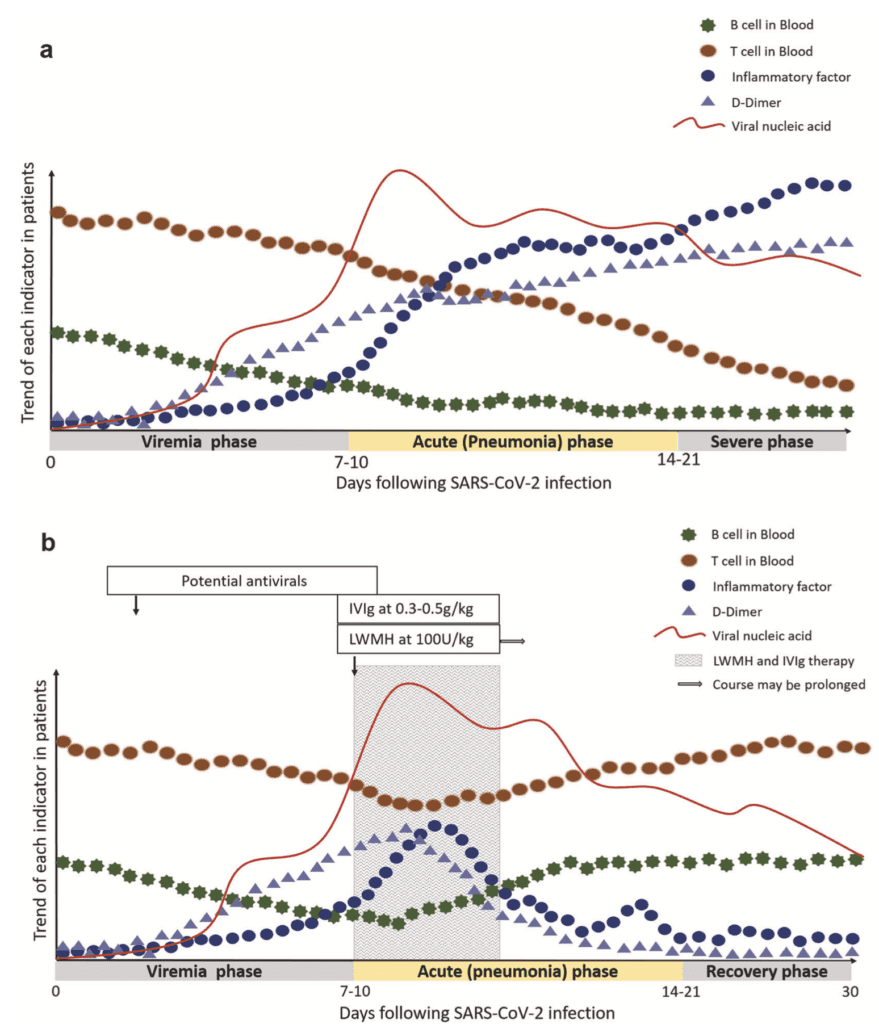
3 Phases of Disease (Viremia, Acute, Severe, Recovery) Over Time vs Trend in T/B cells Inflammatory Factors, D-Dimer, & Viral Load in Patients: A: Severe COVID-19 Without Treatment and B: Severe COVID-19 With Treatment (LMWH & IVIG)
Cytokine Storm [15][16]
- Interleukin-6 (IL-6) is thought to be one of the key inflammatory factors in this syndrome
- Secondary hemophagocytic lymphohistiocystosis (sHLH) is a hyperinflammatory syndrome characterized by fulminant and fatal hypercytokinemia with multiorgan failure
- This is a subset of patients who will have the highest mortality due to a dysregulated immune hyperactivity
- Identifying these patients may help to predict which patients would benefit the most from immunomodulation
Cardinal features:
- Unremitting fever
- Cytopenias
- Hyperferritinemia
- Pulmonary involvement (including ARDS)
Screening for Hyperinflammation:
- Elevated ferritin (Predictor of mortality)
- Decreased platelets
- Elevated ESR
- HScore (Calculator can be found HERE)
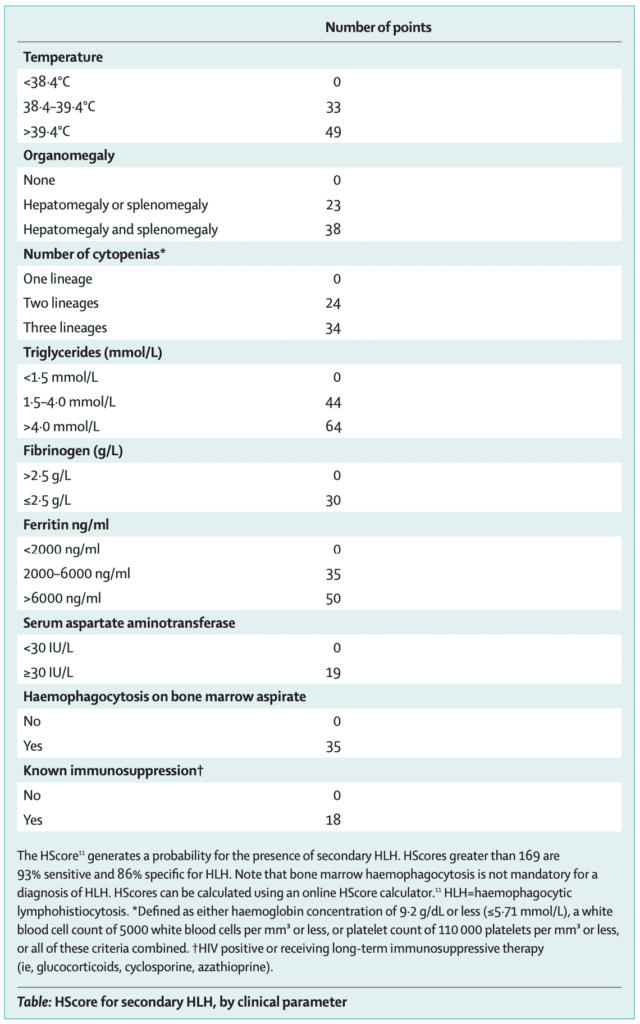
Immunosuppression in these patients may benefit mortality
- Options include:
- Steroids (Although thought to increase viral replication duration, may be of benefit in this select cohort of patients)
- IVIG
- Tocilizumab (IL-6 inhibitor)
Treatment/Prophylaxis for COVID-19 [1]
[embedyt] https://www.youtube.com/watch?v=EXY76TKNy2Y[/embedyt]
COVID-19 Update – March 2020 with Dr. Anthony Fauci, MD from March 18th, 2020 (31:39min)
- There are no approved treatments for any coronavirus currently
- There is no vaccine currently available
- Corticosteroids are not currently recommended and currently under study (NCT04273321)
- Steroids can prolong viral shedding time
- Potential treatments requiring study (Based on animal studies, case reports/series from patients with SARS-CoV and MERS-CoV):
-
Remdesivir:
- Currently under study (Clinicaltrials.gov)
- Activity against Ebola, MERS, and SARS
- Highly effective in one in vitro study [3]
- Case Series of 61 pts with compassionate use [19]
- Gilead, maker of remdesivir funded and wrote manuscript for compassionate use in COVID-19
- Looking for clinical improvement (live discharge from hospital + decrease of at least 2 points from baseline on a modified ordinal scale)
- Modified ordinal scale:
- 1 = not hospitalized
- 2 = hospitalized
- 3 = hospitalized, requiring supplemental oxygen
- 4 = hospitalized, requiring nasal high-flow oxygen therapy, NIV, or both
- 5 = hospitalized, requiring invasive mechanical ventilation, ECMO, or both
- 6 = death
- Pts with O2 sat ≤94% on room air OR receiving O2 support were enrolled
- 10d course of remdesivir (200mg IV d1, then 100mg qd for 9d)
- 61pts received ed at least one dose of remdesivir
- 7 pts had no post-treatment data
- 1 pt had a dosing error
- 53pts analyzed
- 40 (75%) received full 10d course
- Mechanical Ventilation: 30pts (57%)
- ECMO: 4pts (8%)
- Median follow-up of 18d
- Improved O2 Support: 36pts (68%)
- Extubated: 17/30 (57%)
- Discharged Home: 25/53pts (47%)
- Death: 7/53pts (13%)
- Death in pts Receiving Mechanical Ventilation: 6/34 (18%)
- Death in pts NOT Receiving Mechanical Ventilation: 1/19 (5%)
- Limitations:
- No sample size calculation
- No control group
- More authors than patients
- Unblinded trial
- Unclear pt selection
- Many pts not very ill
- Clinical improvement observed in 36/53pts (68%)
- Suggested Dosing: 200mg IV x1d, then 100mg IV qD x9d [Link is HERE]
-
Lopinavir/Ritonavir:
- Currently under study (Clinicaltrials.gov)
- Case Series in JAMA (Published Mar 3, 2020) [2]:
- 5 patients requiring supplemental oxygen treated with lopinavir-ritonavir
- 3 of 5 patents had improved fever and need for supplemental oxygen within 3 days, but 2 deteriorated with progressive respiratory failure
- 4 out 5 patients developed n/v/d
- 3 out 5 patients developed abnormal LFTs
- RCT in NEJM [7]:
- 199 patients with severe COVID-19 Infection
- No difference in time to clinical improvement (primary outcome)
- Improved 28d mortality, ICU LOS, and Percentage of patients with clinical improvement at 14d (All secondary outcomes)
- Stopped early in 13.8% of patients due to adverse events
- REBEL EM Deep Dive [Link is HERE]
- Suggested Dosing: 400-100mg PO BID x 14d [Link is HERE]
-
Chloroquine:
- Anti-Malarial Medication
- Currently under study (Clinicaltrials.gov)
- Highly effective in one in vitro study [3]
- Early Report: Results from >100 patients with COVID-19 demonstrated that chloroquine was superior to control treatment in inhibiting the exacerbation of pneumonia, improving lung imaging findings, promoting a virus-negative conversion, and shortening disease course [4]
- Preliminary double-blinded Phase IIb RCT looking at different doses of Chloroquine (CQ) for treatment of COVID-19 pneumonia performed in Brazil [20]
- Evaluate the safety and efficacy of two different CQ dosages in pts with established severe COVID-19
- Pts randomized to 600mg CQ BID x10d (Total dose 12g) vs CQ 450mg BID x5d (Total Dose 2.7g)
- All pts also received ceftriaxone 1g BID for 7d, azithromycin 500mg qD for 5d, plus oseltamivir 75mg BID for 5d when influenza suspected (89.6% of study population)
- Pre-defined 440 patient sample size, 81 patients enrolled thus far
- Results:
- QTc > 500ms
- Higher Dose CQ = 25% with trend toward higher lethality 17%
- Due to higher lethality rate, prematurely halted patient recruitment to this arm
- Fatality rate = 13.5% (95% CI 6.9 – 23.0%) which overlapped with CI of historical data from similar pts not using CQ (95% CI 14.5 – 19.2%)
- Ongoing larger trial with follow up to 28 days looking to establish long-term lethality
- Respiratory secretion negative for SARS-CoV-2 at day 4 = 1/14pts (7.1%)
- Discussion:
- CQ has been associated with retinal toxicity, myopathy and QTc prolongation resulting in fatal arrhythmias
- Could not use placebo arm, therefore use historical data as comparator
- Limitations:
- Limited sample size recruited thus far does not allow showing benefit regarding treatment efficacy
- Single center study
- Not all cases were COVID-19 confirmed
- Absence of control group using placebo
- Older pts with CAD randomized to high CQ dosage arm
- Bottom Line: Preliminary findings from CloroCovid-19 suggest higher dosage of CQ (12g over 10d) in COVID-19 should not be recommended
- Suggested Dosing: 500mg PO BID x10d [Link is HERE]
-
Tocilizumab:
- Currently under study (Clinicaltrials.gov)
- Binds to IL-6 and blocks it from functioning
- Potentially could help in patients who develop cytokine storm (involves elevated levels of IL-6)
- Suggested Dosing: 8mg/kg in 100mL of 0.9% NS IV over 60min x1 [Link is HERE]
-
Hydroxychloroquine:
- Currently under study (Clinicaltrials.gov)
- Hydroxychloroquine was found to be more potent than chloroquine at inhibiting SARS-CoV-2 in vitro [6]
-
Prospective, observational study (pre-print, not peer reviewed) [Link is HERE]
- 36 hospitalized patients with confirmed COVID-19
- 26 patients got Hydroxychloroquine 200mg PO TID x10d (6 pts also got azithromycin 500mg on day 1, 250mg qD next 4 days)
- 16 patients were controls (no treatment). Patients who refused treatment or had exclusion criteria were controls
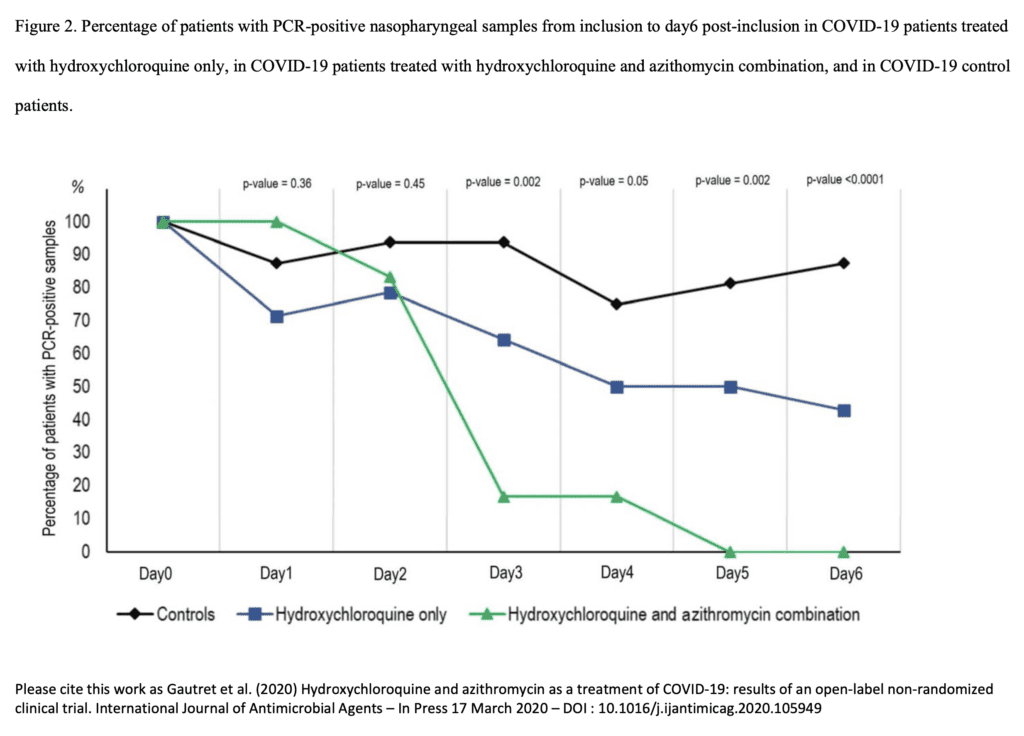
- On day 6 after enrollment 70% of tx group were virologically cured compared to 12.5% in control group
- Comparing hydroxychloroquine alone to hydroxychloroquine + azithromycin on day 6 after enrollment 57.1% vs 100% of patients were virologically cured respectively
- No patient oriented outcomes (i.e. mortality)
- Results are promising, but small sample size, limited long-term outcome follow up and 6 pts dropped out from study
-
Remdesivir:
-
-
- Pilot study out of Shanghai [10] randomized 30 patients with COVID-19 to HCQ 400mg qDx5d + conventional treatment vs conventional treatment alone. No difference in their primary outcome of negative conversion rate of COVID-19 nucleic acid in respiratory pharyngeal swab 7d after randomization. There was also no statistical difference in median duration from hospitalization to virus nucleic acid negative conversion, radiological progression. There was slightly more diarrhea and abnormal LFTs with hydroxychloroquine
-
Small RCT out of China (pre-print, not peer-reviewed) [Link is HERE]
- 62 pts with COVID-19 in China
- Randomized to:
- Additional 5d HCQ 400mg/d + Standard Treatment
- Standard Treatment Alone (O2 therapy, antiviral agents, antibacterial agents, IVIG with or without corticosteroids)
- Time to Clinical Recovery at 6d
- Cough: 2.0d vs 3.1d
- Temperature Recovery: 2.2d vs 3.2d
- Improved PNA: 80.6% vs 54.8%
- 2 pts with mild adverse reactions in HCQ group
- Issues: Small, non-blinded w/ subjective outcomes. Patients also got steroids and IVIG
-
80 patients with COVID-19 in France (pre-print, not peer reviewed) [Link is HERE]
- Received combination of HCQ 200mg TID x10d + AZT 500mg d1 followed by 250mg d2 – d4
- Clinical improvement in 78/80 pts
- Virus cultures negative in 97.5% of pts at day 5
- Issues: No control group
-
1061 pts in France with COVID-19 [Link is HERE]:
- Abstract only
- Single arm observational trial
- Pts treated at least 3d with HCQ-AZT combo + follow up at least 9d
- No cardiac toxicity
- Good “clinical outcome” + Virological cure = 973pts within 10d (91.7%)
-
Observational trial from 4 French hospitals with COVID-19 PNA + Requiring O2 ≥2L/min [22]
- Hydroxychloroquine (HCQ) 600mg/day compared to no HCQ (not randomized)
- Composite primary endpoint transfer to ICU within 7d from inclusion and/or death
- 181 pts
- HCQ within 48hrs of admission = 84pts (17 pts – 20% received concomitant azithromycin + 64 – 76% received concomitant amoxicillin/clavulanic acid)
- No HCQ = 97 pts
- Transfer to ICU or Dead within 7d
- HCQ = 20.2%
- No HCQ = 22.1%
- RR 0.91; 95% CI 0.47 to 1.80
- Death within 7d
- HCQ 2.8%
- No HCQ 4.6%
- RR = 0.61; 95% CI 0.13 to 2.89
- Development of ARDS within 7d
- HCQ = 27.4%
- No HCQ = 24.1%
- RR 1.14; 95% CI 0.65 to 2.0
- 8/84 pts (9.5%) developed ECG abnormalities requiring HCQ discontinuation
- Discussion:
- In this trial pts did not receive other anti-viral and anti-inflammatory treatments including steroids
- Many patients had CRP > 40mg/L, which may suggest that a cytokine storm had already begun (90.5% in HCQ group and 81.9% in no HCQ group)
- Limitations:
- Not an RCT meaning there may be unmeasured confounders
- Limited number of patients with mortality
- HCQ at different hospitals was unbalanced (some centers treated all pts with HCQ, while others did not)
- Bottom Line: HCQ added to standard care was not associated with reduction of admissions to ICU or death 7d after hospital admission compared to standard care alone
-
Multicenter, parallel, open-label randomized trial of 150 adult patients hospitalized for COVID-19 in China [23]
- HCQ with loading dose of 1200mg qd for 3d, followed by maintenance dose of 800mg for remaining days (total treatment duration 2 – 3 weeks) + standard care
- Standard care alone
- Primary endpoint = 28 negative conversion rate of SARS-CoV-2
- HCQ: 85.4%
- No HCQ: 81.3%
- P = 0.341
- Negative conversion rate at day 4, 7, 10, 14, or 21 was similar between groups
- No difference in 28d symptom alleviation rate between groups, but more rapid alleviation of clinical symptoms with HCQ (2nd week of illness)
- Initial CRP levels and lymphopenia normalized early, but overall improvement rate was not different at 28d
- Adverse events:
- HCQ: 30%
- No HCQ: 8.8%
- Serious Adverse Events: 2 (2.9%) HCQ vs 0 (0%) standard care alone
- Most common adverse event in HCQ group = diarrhea (10%)
- Limitations:
- No patient-oriented outcomes (i.e. mortality)
- Unblinded study which can bias subjective outcomes of symptoms
- Most patients with mild to moderate disease (i.e. only 1% with severe disease)
- Trial terminated early
- Bottom Line: HCQ did not result in higher negative conversion rate but had more alleviation of clinical symptoms compared to standard care alone
-
Retrospective observational analysis of 368pts evaluated from US Veterans Health Administration [25]
- HCQ Alone = 97pts
- HCQ + AZT = 113pts
- No HCQ = 158pts
- Rate of Death:
- HCQ Alone = 27.8%
- HCQ + AZT = 22.1%
- No HCQ = 11.4%
- Need for Mechanical Ventilation:
- HCQ Alone = 13.3%
- HCQ + AZT = 6.9%
- No HCQ = 14.1%
- Bottom Line: HCQ with or without AZT did not reduce risk of mechanical ventilation with an association of increased overall mortality compared to no HCQ
-
Observational trial of 1376 patients [28]
- Not associated with lowered or increased risk of composite endpoint of intubation or death
-
Retrospective observational Multicenter trial of 1438 patients treated with HCQ and/or AZT [29]
- No difference in in-hospital mortality
-
RCT of 150 patients in China [30]
- HCQ + Standard Care vs Standard Care Alone
- No difference in conversion to negative RT-PCR of SARS-CoV-2
- Adverse events: HCQ 30% vs Standard Care Alone 9%
- Most common adverse event was diarrhea
- Suggested Dosing: 400mg PO BID x1d, then 200mg PO BID x4d [Link is HERE]
-
Favipiravir:
- Currently under study [Clinicaltrials.gov]
- Suggested Dosing: 1600mg PO BID x1d, then 600mg PO BID x6d [Link is HERE]
-
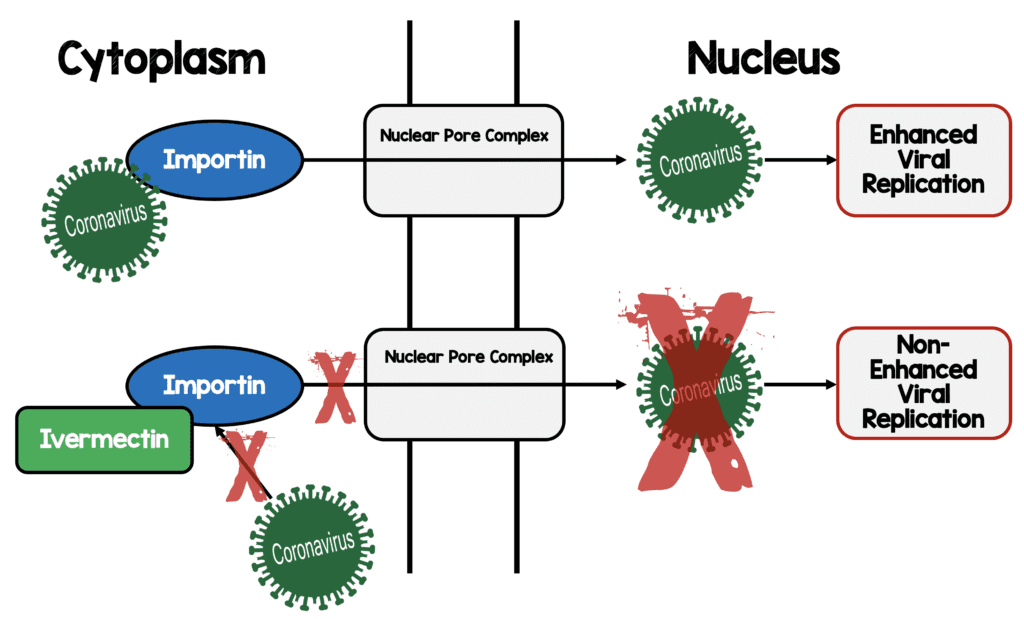
Ivermectin [17]:
- In vitro study of SARS-CoV-2 & single dose ivermectin
- 24h = 93% reduction in viral RNA present in supernatant
- 24h = 99.8% reduction in cell-associated viral RNA
- 48h = ≈5000-fold reduction of viral RNA in ivermectin-treated vs control samples
- No toxicity observed at anytime
- Nuclear transport inhibitory activity
- In vitro study of SARS-CoV-2 & single dose ivermectin
-
-
-
Convalescent Plasma [18]:
- 5 critically ill patients with COVID-19 and ARDS with severe pneumonia, high viral load, PaO2/FiO2 <300, and mechanical ventilation
- Pts received transfusion with convalescent plasma with a SARS-CoV-2 specific antibody (IgG) on days 10 and 22 after admission
- SOFA score decreased
- PaO2/FiO2 improved in 4/5 pts
- Body temp declined to normal by 3rd day
- CRP, Procalcitonin, and IL-6 decreased in 4/5 pts
- IgG and IgM titers increased & remained elevated for 7d post-transfusion
- 3/5 pts weaned from mechanical ventilation
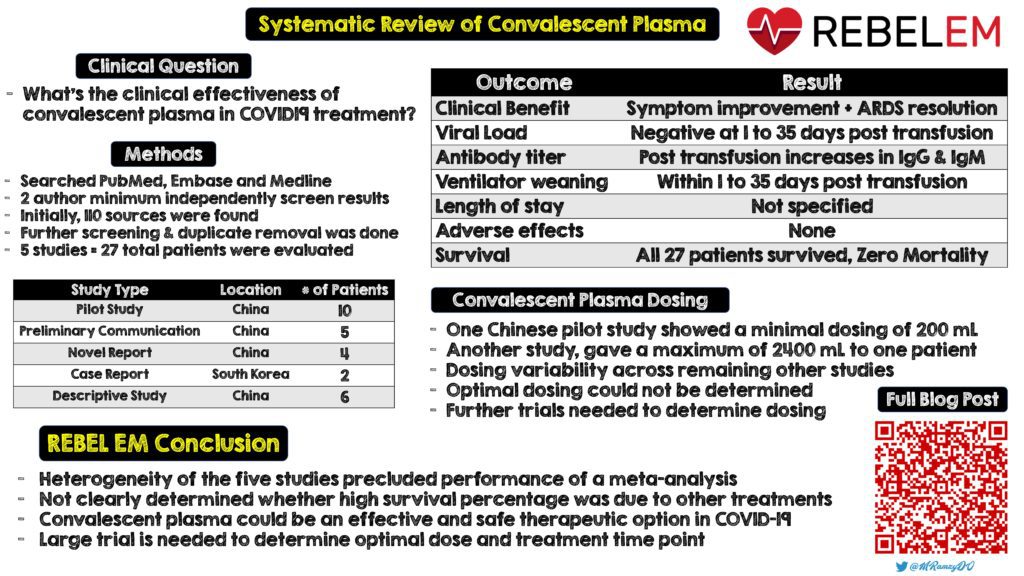
- Systematic review of 5 trials with 27 patients [27]
- 5/5 studies found significant reductions in viral load and increased neutralizing antibody over time
- Almost all patients showed improvements in body temperature, lung lesions, ARDS resolution, weaning from mechanical ventilation
- 5/5 studies reported zero mortality
- No adverse events occurred
- 5 critically ill patients with COVID-19 and ARDS with severe pneumonia, high viral load, PaO2/FiO2 <300, and mechanical ventilation
-
Convalescent Plasma [18]:
Infographic Created By Mark Ramzy, DO (Twitter: @MRamzyDO)
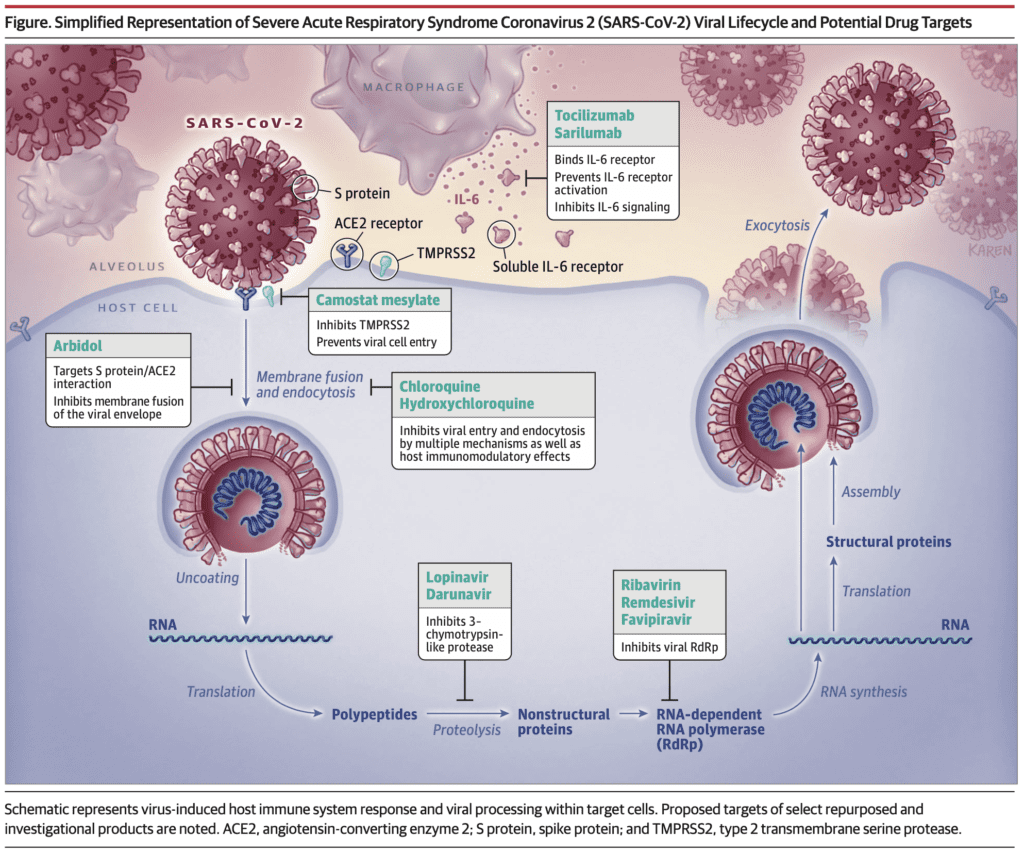
Potential Drug Targets from JAMA [21]
ACEI/ARBs and COVID19 [5][11][13]:
- SARS-CoV-2 gains entry to pulmonary cells after binding to membrane ACE2
- ACE1 converts angiotensin I to angiotensin II
- ACE2 converts angiotensin II to angiotensin
- Angiotensin II is the known substrate for ACE2
- Plasma levels of angiotensin II increase with ARB dosing
- ACEI reduce concentrations of angiotensin II
- Increased ACE2 expression with ACEI/ARBs has been shown in the kidneys and heart, but not tested in the lungs
- Use of ACEI/ARBs could increase ACE2 expression and therefore may increase human SARS-CoV-2 infectivity and severity of illness
- Data in humans is limited at this time to support or refute the concerns with ACEI/ARBs
- Population-based studies out of China estimate that only 30 to 40% of patient have HTN treated with any antihypertensive therapy and RAAS inhibitors are used only in 25 to 30% of these treated patients [14].
- There is conflicting preclinical models and animal data on the benefits and harms of ACEI/ARBs, but these may not translate to human physiology
- Recommendation to discontinue ARBs should not be common practice until confirmation of this hypothesis is as this can result in elevation of BP, which might occur with treatment changes, and carries risks which are not hypothetical
-
The European Society of Cardiology has a position statement on this from 03/13/2020 [Link is HERE]:
- HTN may be associated with increased risk of mortality in hospitalized COVID-19 infected pts
- Hypotheses put forward suggest a potential adverse effect of ACEI or ARBs
- Concern arises from plausible observation that, COVID19 virus binds to ACE2 to infect cells, and ACE2 levels are increased following ACEI/ARB
- There is some animal data suggesting that these meds might be protective against serious lung complications in patients with COVID19 infection but to date there is no data in humans
- There is no sound scientific basis or evidence to support this claim
- “The Council on Hypertension strongly recommend that physicians and patients should continue treatment with their usual anti-hypertensive therapy because there is no clinical or scientific evidence to suggest that treatment with ACEI/ARBs should be discontinued because of the COVID-19 infection.”
-
The HFSA/ACC/AHA Statement has a position statement on this from 03/17/2020 [Link is HERE]:
- The role of ACE2 in the setting of COVID-19 infection in patients with cardiovascular disease is unclear
- Currently there is no experimental or clinical data demonstrating beneficial or adverse outcomes with background use of ACEI, ARBs, or other RAAS antagonists in COVID-19
- They recommend continuation of RAAS antagonists for those patients who are currently prescribed these medications and to not add or remove any RAAS-related medications, beyond actions based on standard clinical practice
-
Association of Inpatient Use of Angiotensin Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers with Mortality Among Patients with Hypertension Hospitalized with COVID-19 [24]
- Determine the association between in-hospital use of ACEI/ARB and all-cause mortality in COVID-19 patients with hypertension admitted to 9 hospitals in China
- Retrospective, multicenter study of 1128 adult patients with HTN diagnosed with COVID-19:
- Taking ACEI/ARB: 188
- Not Taking ACEI/ARB: 940
-
Unadjusted 28d Mortality:
- No ACEI/ARB: 9.8%
- ACEI/ARB: 3.7%
- P = 0.01
- uaHR: 0.42; 95% CI 0.19 to 0.92; p = 0.03
- aHR: 0.37; 95% CI 0.15 to 0.89; p = 0.03
- ACEI/ARB also associated with decreased mortality compared to other antihypertensive drugs (aHR 0.30; 95% CI 0.12 to 0.70; p = 0.01
- Bottom Line: Inpatient use of ACEI/ARB was associated with lower risk of all-cause 28d mortality compared with ACEI/ARB non-users. Results were matched and adjusted for multiple variables and still showed an association with reduced all-cause mortality in patients with HTN
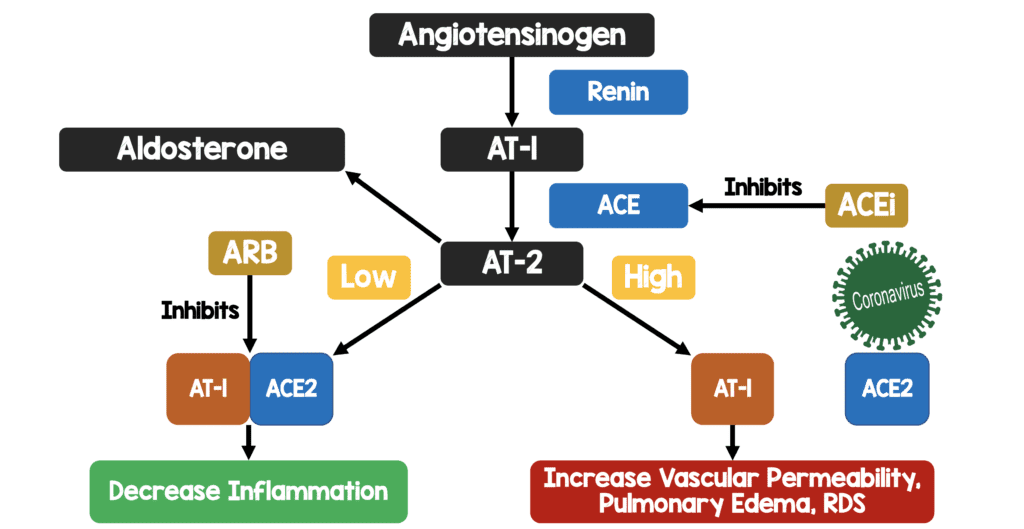
My Oversimplified Schematic of ACE2 and COVID19 Infection Theory
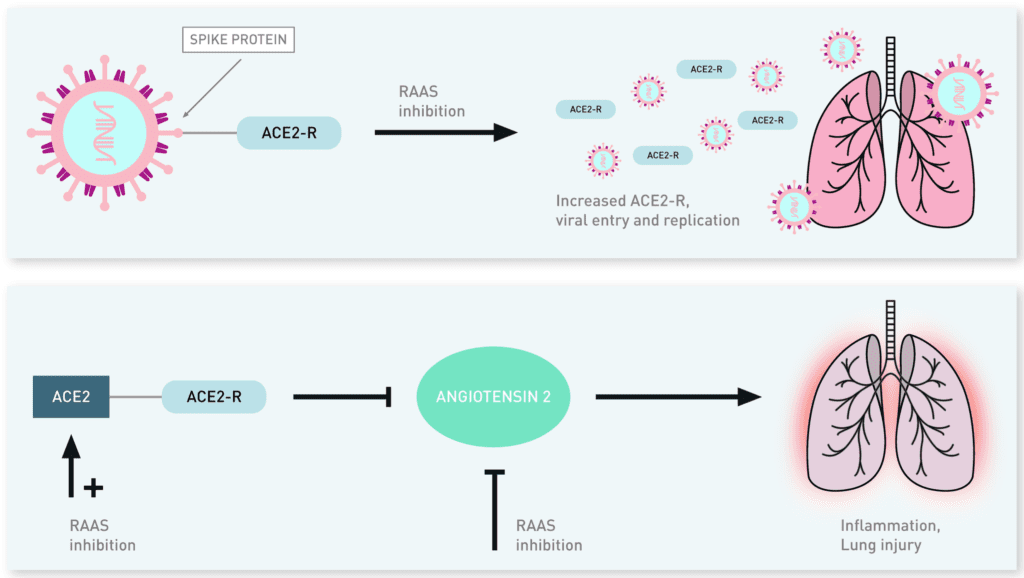
Potential Mechanisms for ACE2 with Regard to Viral Pathogenicity and Lung Protection [9]
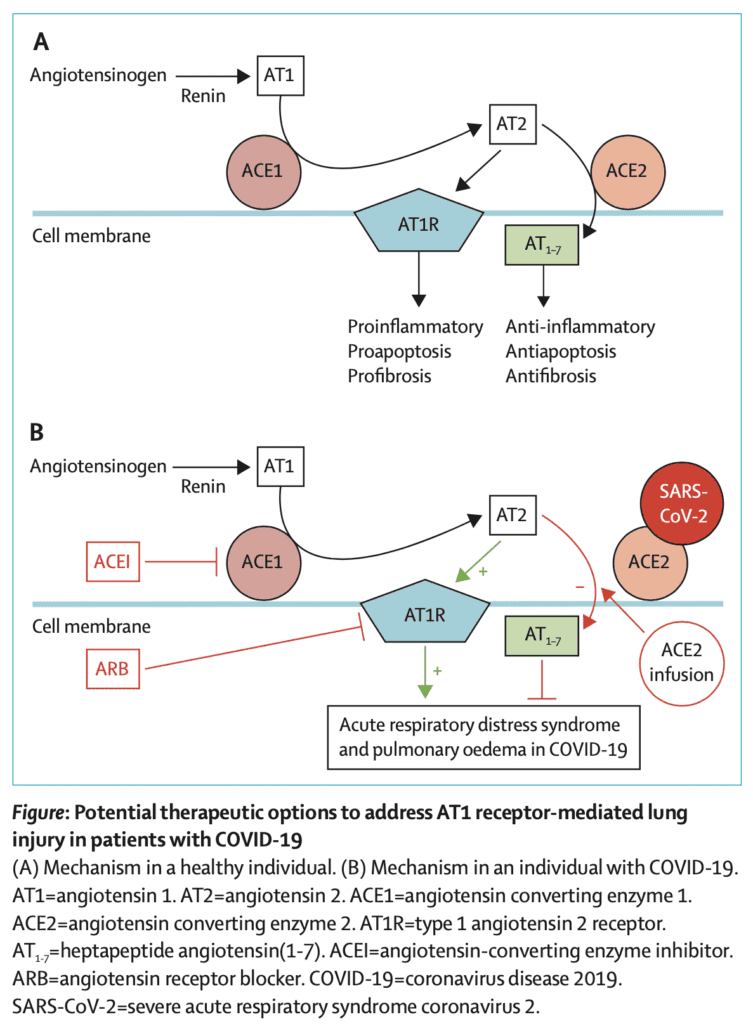
Potential Therapeutic Options to Address AT1 Receptor-Mediated Lung Injury in Patients with COVID-19 (A: Mechanism in Healthy Pts; B: Mechanism in Pts with COVID-19)[13]
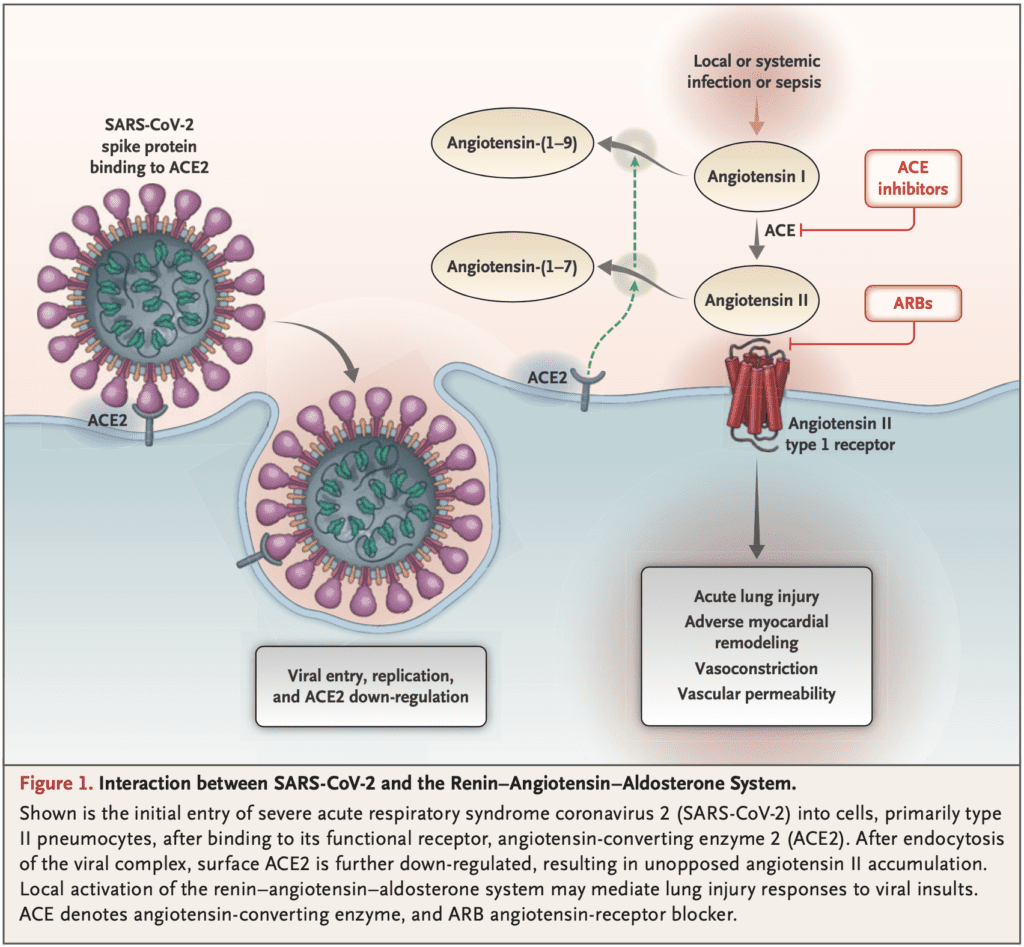
Interaction Between SARS-CoV-2 and the Renin-Angiotensin-Aldosterone System [14]
Ibuprofen:
- Reports out of France indicate that Ibuprofen worsens the effects of COVID19 (See tweet below)
- A report in the Lancet (Link is HERE) stated that Ibuprofen up-regulates expression of ACE2 (The binding site of SARS-CoV-2)
- Although there is a biologically plausible explanation that ACE2 expression is increased with the use of Ibuprofen, there is NO robust evidence at this time that shows worsened outcomes in COVID19 infection. Until that time, using acetaminophen as the first line anti-pyretic in patients infected with COVID19. Ibuprofen may or may not cause worsened outcomes as it is an observation that would need a larger trial to give causation. Is it possible that these patients were sicker and thats why we see worsened outcomes?
#COVIDー19 | La prise d'anti-inflammatoires (ibuprofène, cortisone, …) pourrait être un facteur d'aggravation de l’infection. En cas de fièvre, prenez du paracétamol.
Si vous êtes déjà sous anti-inflammatoires ou en cas de doute, demandez conseil à votre médecin.— Olivier Véran (@olivierveran) March 14, 2020
Q: Could #ibuprofen worsen disease for people with #COVID19?
A: Based on currently available information, WHO does not recommend against the use of of ibuprofen. pic.twitter.com/n39DFt2amF
— World Health Organization (WHO) (@WHO) March 18, 2020
Why Off-Label Drug Use, Compassionate Use Don’t get us Answers [12]
- Single group interventions without concurrent controls lead to no definitive conclusions related to efficacy or safety
- Administration of any unproven drug as a “last resort” wrongly assumes that benefit will be more likely than harm
- Compassionate use of drugs that have not been studied could cause serious adverse effects
- “The rapid and simultaneous combination of supportive care and RCTs is the only way to find effective and safe treatments for COVID-19 and any other future outbreak”
References:
- Young BE et al. Epidemiologic Features and Clinical Course of Patients Infected with SARS-CoV-2 in Singapore. JAMA 2020. [Epub Ahead of Print]
- Wang M et al. Remdesivir and Chloroquine Effedtively Inhibit the Recently Emerged Novel Coronavirus (2019-nCoV) in Vitro. Cell Res 2020. PMID: 32020029
- Gao J et al. Breakthrough: Chloroquine Phosphate has Shown Apparent Efficacy in Treatment of COVID-19 Associate Pneumonia in Clinical Studies. Biosci Trends 2020. PMID: 32074550
- Murray E et al. Can Angiotensin Receptor-Blocking Drugs Perhaps be Harmful in the COVID-19 Pandemic?. Journal of Hypertension 2020. [Epub Ahead of Print]
- X Y et al. In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Clinical Infect Dis 2020. PMID: 32150618
- Cao B et al. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe COVID-19. NEJM 2020. [Epub Ahead of Print]
- Siddiqi HK et al. COVID-19 Illness in Native and Immunosuppressed States: A Clinical-Therapeutic Staging Proposal. J Heart and Lung Transplantation 2020. [Epub Ahead of Print]
- Driggin E et al. Cardiovascular Considerations for Patients, Health Care Workers, and Health Systems During the Coronavirus Disease 2019 (COVID-19) Pandemic JACC 2020. [Epub Ahead of Print]
- Driggin E et al. Cardiovascular Considerations for Patients, Health Care Workers, and Health Systems During the Coronavirus Disease 2019 (COVID-19) Pandemic JACC 2020. [Epub Ahead of Print]
- Jun C et al. A Pilot Study of Hydroxychloroquine in Treatment of Patients with Common Coronavirus Disease-19 (COVID-19). J Zhejiang Univ (Med Sci) 2020. [Epub Ahead of Print]
- Patel AB et al. COVID-19 and Angiotensin-Converting Enzyme Inhibitors and Angiotensin Receptor Blocker: What is the Evidence? JAMA 2020. [Epub Ahead of Print]
- Kalil AC. Treating COVID-19-Off-Label Drug Use, compassionate Use, and Randomized Clinical Trials During Pandemics. JAMA 2020. [Epub Ahead of Print]
- Tignanelli CJ et al. Antihypertensive Drugs and Risk of COVID-19? Lancet Respir Med 2020 [Epub Ahead of Print]
- Vaduganathan M et al. Renin-Angiotensin-Aldosterone System Inhibitors in Patients with COVID-19. NEJM 2020. [Epub Ahead of Print]
- Zhang Chi et al. The Cytokine Release Syndrome (CRS) of Severe COVID-19 and Interleukin-6 Receptor (IL-6R) Antagonist Tocilizumab man be the Key to Reduce the Mortality. Int J Antimicrob Agents 2020. PMID: 32234467
- Mehta P et al. COVID-19: Consider Cytokine Storm Syndromes and Immunosuppression. Lancet 2020. PMID: 32192578
- Caly L et al. The FDA-Approved Drug Ivermectin Inhibits the Replication of SARS-CoV-2 in Vitro. Antiviral Research 2020. [Epub Ahead of Print]
- Shen C et al. Treatment of 5 Critically Ill Patients With COVID-19 With Convalescent Plasma. JAMA 2020. PMID: 32219428
- Grein J et al. Compassionate Use of Remdesivir for Patients with Severe COVID-19. NEJM 2020. [Epub Ahead of Print]
- Silva Borba MG et al. Chloroquine Diphosphate in Two Different Dosages as Adjunctive Therapy of Hospitalized Patients with Severe Respiratory Syndrome in the Context of Coronavirus (SARS-CoV-2) Infection: Preliminary Safety Results of a Randomized, Double-Blinded, Phase IIb Clinical Trial (CloroCovid-19 Study). MedRxiv Preprint [Epub Ahead of Print]
- Sanders JM et al. Pharmacologic Treatments for Coronavirus Disease 2019 (COVID-19) A Review. JAMA 2020. [Epub Ahead of Print]
- Mahevas M et al. No Evidence of Clinical Efficacy of Hydroxychloroquine in Patients Hospitalised for COVID-19 Infection and Requiring Oxygen: Results of a Study Using Routinely collected Data to Emulate a Target Trial. MedRxiv Preprint 2020 [Epub Ahead of Print]
- Tang W et al. Hydroxychloroquine in Patients with COVID-19: An Open-Label, Randomized, Controlled Trial. MedRxiv Preprint 2020. [Epub Ahead of Print]
- Zhang P et al. Association of Inpatient Use of Angiotensin Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers with Mortality Among Patients with Hypertension Hospitalized with COVID-19. Circ Res 2020. PMID: 32302265
- Magagnoli J et al. Outcomes of Hydroxychloroquine Usage in United States Veterans Hospitalized with COVID-19. medRxiv 2020 Preprint, Non-Peer Reviewed [Epub Ahead of Print]
- Cao W et al. COVID-19: Towards Understanding Pathogenesis. Cell Research 2020. [Epub Ahead of Print]
- Rahendran K et al. Convalescent Plasma Transfusion for the Treatment of COVID-19: Systematic Review. J Med Virol 2020. PMID: 32356910
- Geleris J et al. Observational Study of Hydroxychloroquine in Hospitalized Patients with COVID-19. NEJM 2020. [Epub Ahead of Print]
- Rosenberg ES et al. Association of Treatment With Hydroxychloroquine or Azithromycin With In-Hospital Mortality in Patients With COVID-19 in New York State. JAMA 2020. PMID: 32392282
- Tang W et al. Hydroxychloroquine in Patients with Mainly Mild to Moderate Coronavirus Disease 2019: Open Label, Randomized Controlled Trial. BMJ 2020. PMID: 32409561
- Mehra MR et al. Hydroxychloroquine or Chloroquine With or Without a Macrolide for Treatment of COVID-19: A Multinational Registry Analysis. Lancet 2020. [Epub Ahead of Print]
For More Thoughts on This Topic Checkout:
- REBEL EM: COVID-19 – The Novel Coronavirus 2019
- FOAMCast: High Flow Nasal Cannula, Cytokine Storm, Asymptomatic Transmission
- CDC: Information for Clinicians on Therapeutic Options for COVID-19 Patients
- DFTB: COVID and ACE Inhibitors
- First10EM: Chloroquine for COVID – No Good Evidence Yet
- EM Lit of Note: Update on Hydroxychloroquine and Azithromycin
- emDocs: Antiviral Agents: What is Their Use in COVID-19?
- First10EM: Antivirals for COVID19 – Not Ready for Clinical Use
- emDocs: Anti-Inflammatory Agents and Corticosteroids in COVID-19: What’s the Controversy?
- emDocs: Chloroquine & Hydroxychloroquine – Is There Value in COVID-19?
- PulmCrit: 11 Reasons the NEJM Paper on Remdesivir Reveals Nothing
- PulmCrit: Hydroxychloroquine Fails First Meaningful RCT
- PulmCrit: Timed and Titrated Use of Steroid in COVID-19?
- emDocs: The Storm Within – Cytokine Storm and COVID-19
- PulmCrit: Before/After Study of short-Course Steroid in COVID-19
Post Peer Reviewed By: Anand Swaminathan, MD (Twitter: @EMSwami) and Mizuho Morrison, DO (Twitter: mizuhomorrison)
The post COVID-19: Clinical/Therapeutic Staging Proposal and Treatment appeared first on REBEL EM - Emergency Medicine Blog.