 The Novel Coronavirus 2019, was first reported on in Wuhan, China in late December 2019. The outbreak was declared a public health emergency of international concern in January 2020 and on March 11th, 2020, the outbreak was declared a global pandemic. The spread of this virus is now global with lots of media attention. The virus has been named SARS-CoV-2 and the disease it causes has become known as coronavirus disease 2019 (COVID-19). This new outbreak has been producing lots of hysteria and false truths being spread, however the data surrounding the biology, epidemiology, and clinical characteristics are growing daily, making this a moving target. This post will serve as a summary of screening, testing, patients under investigation (PUI), and returning to work in regards to COVID-19.
The Novel Coronavirus 2019, was first reported on in Wuhan, China in late December 2019. The outbreak was declared a public health emergency of international concern in January 2020 and on March 11th, 2020, the outbreak was declared a global pandemic. The spread of this virus is now global with lots of media attention. The virus has been named SARS-CoV-2 and the disease it causes has become known as coronavirus disease 2019 (COVID-19). This new outbreak has been producing lots of hysteria and false truths being spread, however the data surrounding the biology, epidemiology, and clinical characteristics are growing daily, making this a moving target. This post will serve as a summary of screening, testing, patients under investigation (PUI), and returning to work in regards to COVID-19.
To go back to the main post, click on the image below…
Screening for COVID-19
- Follow your institutional protocols for identifying high risk patients from triage and moving them to a negative isolation room expeditiously
The CDC has updated their criteria for screening to include: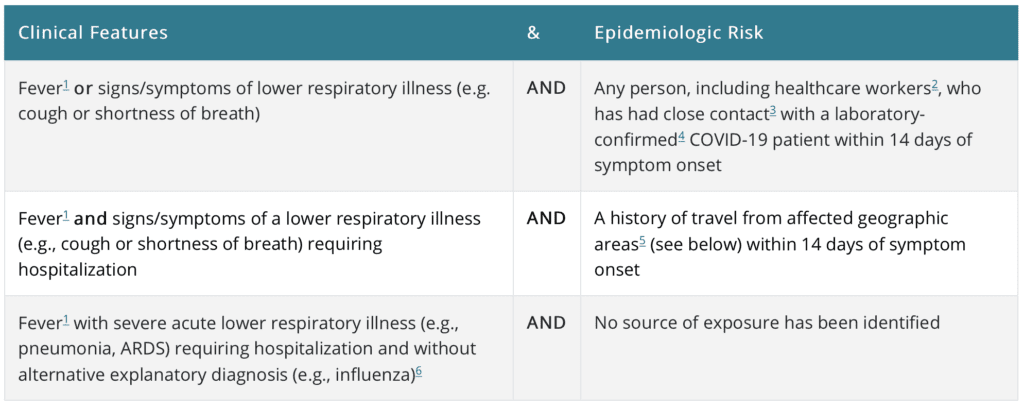 From the CDC Website Mar 1st, 2020
From the CDC Website Mar 1st, 2020
*Affected Geographic Areas with Sustained Transmission per the CDC: China, Iran, Italy, Japan, South Korea
**Close Contact per the CDC: Being within ≈6ft (2m) of a COVID-19 case for a prolonged period of time; close contact can occur while caring for , living with, visiting, or sharing a healthcare eating area or room with a COVID-19 case OR having direct contact with infectious secretions of a COVID-19 case (i.e. being coughed on) without personal protective equipment (PPE)
This may not be the best strategy: REBEL EM – Why COVID-19 Screening Protocols Won’t Work
Testing for COVID-19 – Persons Under Investigation – PUI (Updated March 18th, 2020)
- Mildly ill patients would be encouraged to stay home and contact their healthcare provider by phone for guidance about clinical management.
- Patients with severe symptoms, such as difficulty breathing, should seek care immediately
- Report all potential cases of COVID-19 to infection control at your institution, and state/or local health departments
- Widely available respiratory viral panels test only for earlier forms of human coronavirus, BUT not SARS-CoV, MERS-CoV, and COVID-19 strains which require more specialized assays
- Currently, diagnostic testing for COVID-19 is being performed at state public health laboratories and the CDC
- Additionally diagnostic testing, authorized by the FDA is becoming available in clinical laboratories
- Priority testing should include:
- Hospitalized patients who have signs and symptoms compatible with COVID-19 in order to inform decisions related to infection control
- Other symptomatic individuals such as, older adults (age ≥65 years) and individuals with chronic medical conditions and/or an immunocompromised state that may put them at higher risk for poor outcomes (i.e. diabetes, heart disease, receiving immunosuppressive medications, chronic lung disease, CKD)
- Any persons including healthcare personnel, who within 14 days of symptom onset had close contact with a suspect or laboratory-confirmed COVID-19 patient, or who have a history of travel from affected geographic areas within 14 days of their symptom onset
- It is recommended that the specimen submitted for reverse-transcriptase polymerase chain reaction (RT-PCR) testing be nasopharyngeal (NOT a throat swab)
- CDC also recommends a lower respiratory tract sample such as induced sputum or bronchoalveolar lavage. In my opinion, this should not be done as this can aerosolize virus.
- Sensitivity of the RT-PCR test for COVID-19 has been reported as 66 – 80% [20]
- Excluding a diagnosis of COVID-19 will require multiple negative RT-PCR tests (i.e. a single negative RT-PCR DOES NOT exclude COVID-19, especially if taken early in the disease course)
- If RT-PCR is negative but their is a suspicion for COVID-19, then ongoing isolation and re-checking several days later should be considered (i.e. unclear on ideal window ≈24 – 72hrs?)
- It is important to remember that patients with pneumonia due to COVID-19 may have lung abnormalities on chest CT or lung US and an initially negative RT-PCR
- Sensitivity of RT-PCR in small retrospective case series of 25 patients was 70.6% [4]
-
Just to muddy the waters a bit more on testing:
- Study out of Stanford [Link is Here], of the 49 positive SARS-CoV-2 results, 11 (22.4%) also had a co-infection
- 21 patients positive for COVID-19 infection [2]
-
Evidence of Co-Infection:
- Bacterial: 4.8%
- Viral: 14.3%
[embedyt] https://www.youtube.com/watch?v=oGiOi7eV05g[/embedyt]
Coronavirus Testing with Jay Butler, MD from JAMANetwork (Published March 16th, 2020) – Total Time 37:24min
-
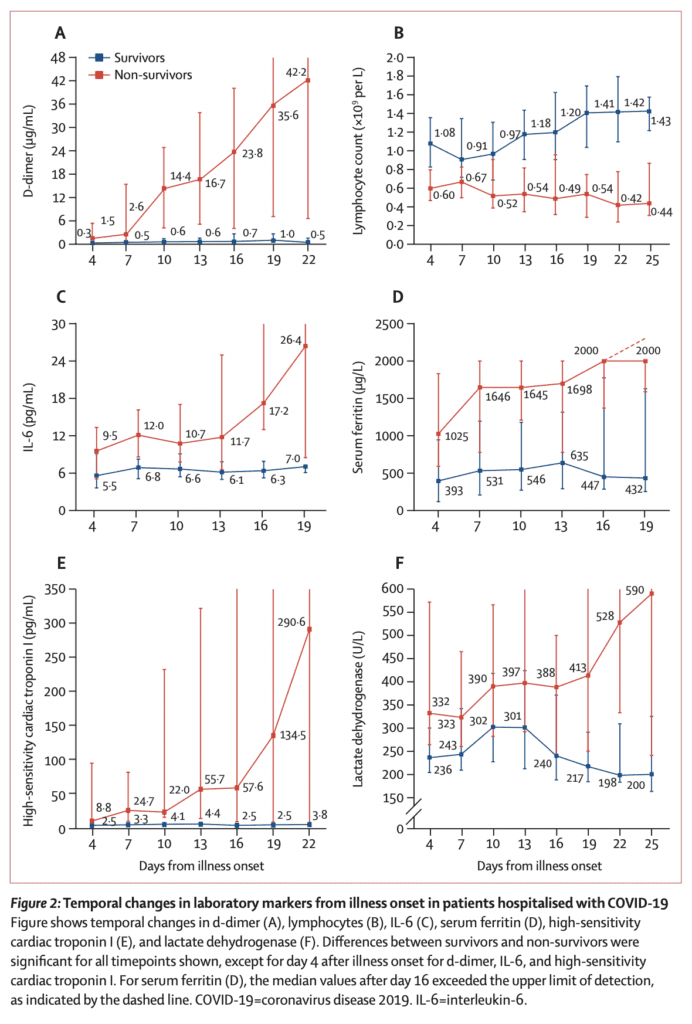
Severity of Disease Correlates with Viral Load and Longer Viral Shedding Time [1]:
- 76 patients admitted with confirmed COVID-19 infection divided into mild (46 pts) and severe (30 pts) cases
- Severe cases defined as:
- Respiratory distress (≥30BPM)
- O2 saturation at rest ≤93%
- P/F ratio ≤300mmHg
- Severe disease complications (i.e. respiratory failure, need for mechanical ventilation, septic shock, or non-respiratory organ failure)
- Mild cases found to have early viral clearance, with 90% of patients repeatedly testing negative on RT-PCR by day 10 post-onset, whereas severe cases still tested positive at ≥10 days post onset
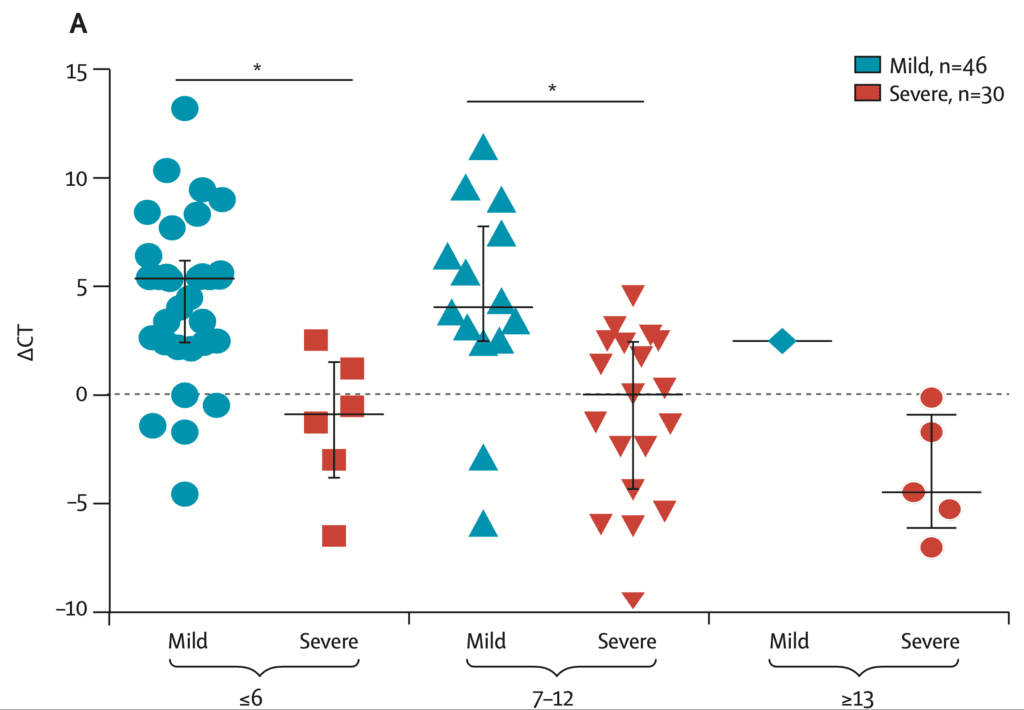
ΔCt Values of Severe Cases Over Time in Days
-
- Viral loads measured with nasopharyngeal swab samples with a ΔCt method (Ctsample – Ctref)
- Therefore a larger ΔCt value correlates with a smaller viral load and a smaller ΔCt value correlates with a larger viral load
-
Characteristics and Outcomes of 21 Critically Ill Patients with COVID-19 in Washington State[2]:
- This is a description of the clinical presentation, characteristics and outcomes of 21 COVID-19 patients admitted to a single center in Washington
- Mean Age: 70 years (Range: 43 – 92)
- Comorbidities Present: 86% (i.e. CKD, CHF, DM)
- Initial Symptoms:
- Shortness of Breath: 76.2%
- Fever: 52.4%
- Cough: 47.2%
- Mean Symptom Onset Prior to Presenting to Hospital: 3.5d
- Admit to ICU Within 24hrs After Admission: 81.0%
- Abnormal CXR Findings:
- Bilateral reticular nodular opacities: 52.4%
- Ground-glass opacities: 47.6%
- Labs:
- Absolute Lymphocyte Count (<1000 cells/uL): 67%
- Abnormal LFTs: 38%
- ARDS:
- None: 4.8%
- Mild: 9.5%
- Mod: 28.6%
- Severe: 57.1%
- Required Mechanical Ventilation: 71%
-
Evidence of Co-Infection:
- Bacterial: 4.8%
- Viral: 14.3%
- Cardiomyopathy: 33.3%
- Outcomes:
- Death: 52.4%
- Survived to Transfer out of ICU: 9.5%
- Limitations: Small number of patients, single center, older residents from skilled nursing facility will limit application to broader patient population
-
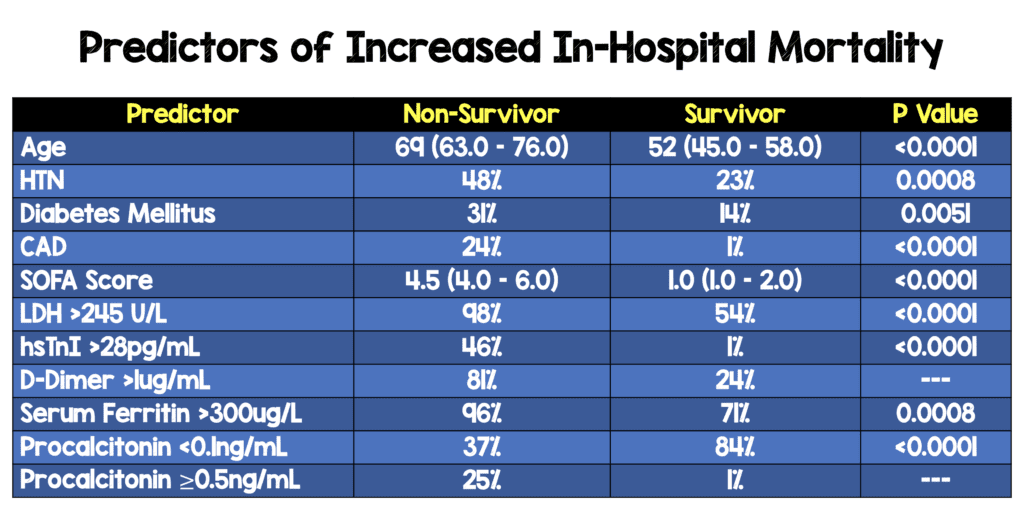
Predictors of Increased In-Hospital Mortality[3]:
- Retrospective, multicenter cohort study
- 191 patients from China with COVID-19
- 137 patients were discharged home
- 54 patients died
A Comment on Testing
- Although we don’t know the true sensitivity and specificity of the RT-PCR assay, a key important point is that we ensure we are getting a good sample. This is a sample swabbed from the back of the nose. This may be uncomfortable for patients but necessary. A anterior nasal swab will just not cut it.
Patients Under Home Isolation as of March 16th, 2020 (From the CDC Website):
The decision to discontinue home isolation should be made in the context of local circumstances. Options now include both 1) a time-since-illness-onset and time-since-recovery (non-test-based) strategy, and 2) a test-based strategy.
-
Time-since-illness-onset and time-since-recovery strategy (non-test-based strategy)*
Persons with COVID-19 who have symptoms and were directed to care for themselves at home may discontinue home isolation under the following conditions:- At least 3 days (72 hours) have passed since recovery defined as resolution of fever without the use of fever-reducing medications and improvement in respiratory symptoms (e.g., cough, shortness of breath); and,
- At least 7 days have passed since symptoms first appeared.
- Test-based strategy (simplified from initial protocol) Previous recommendations for a test-based strategy remain applicable; however, a test-based strategy is contingent on the availability of ample testing supplies and laboratory capacity as well as convenient access to testing. For jurisdictions that choose to use a test-based strategy, the recommended protocol has been simplified so that only one swab is needed at every sampling.
-
Persons who have COVID-19 and who have symptoms and were directed to care for themselves at home may discontinue home isolation under the following conditions:
- Resolution of fever without the use of fever-reducing medications and
- Improvement in respiratory symptoms (e.g., cough, shortness of breath) and
- Negative results of an FDA Emergency Use Authorized molecular assay for COVID-19 from at least two consecutive nasopharyngeal swab specimens collected ≥24 hours apart** (total of two negative specimens). See Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens from Persons Under Investigation (PUIs) for 2019 Novel Coronavirus (2019-nCoV)for specimen collection guidance.
- Individuals with laboratory-confirmed COVID-19 who have not had any symptoms may discontinue home isolation when at least 7 days have passed since the date of their first positive COVID-19 diagnostic test and have had no subsequent illness.
-
Footnote
- *This recommendation will prevent most, but may not prevent all instances of secondary spread. The risk of transmission after recovery, is likely very substantially less than that during illness.
- **All test results should be final before isolation is ended. Testing guidance is based upon limited information and is subject to change as more information becomes available.
Returning to Work for Healthcare Providers as of April 30th, 2020 (From the CDC Website ):
-
Symptomatic HCP with suspected or confirmed COVID-19:
- Symptom-based strategy. Exclude from work until:
-
- At least 3 days (72 hours) have passed since recovery defined as resolution of fever without the use of fever-reducing medications and improvement in respiratory symptoms (e.g., cough, shortness of breath); and,
- At least 10 days have passed since symptoms first appeared
- Test-based strategy. Exclude from work until:
-
- Resolution of fever without the use of fever-reducing medications and
- Improvement in respiratory symptoms (e.g., cough, shortness of breath), and
- Negative results of an FDA Emergency Use Authorized COVID-19 molecular assay for detection of SARS-CoV-2 RNA from at least two consecutive nasopharyngeal swab specimens collected ≥24 hours apart (total of two negative specimens). See Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens for 2019 Novel Coronavirus (2019-nCoV). Of note, there have been reports of prolonged detection of RNA without direct correlation to viral culture.
HCP with laboratory-confirmed COVID-19 who have not had any symptoms:
-
Time-based strategy. Exclude from work until:
- 10 days have passed since the date of their first positive COVID-19 diagnostic test assuming they have not subsequently developed symptoms since their positive test. If they develop symptoms, then the symptom-basedor test-based strategy should be used. Note, because symptoms cannot be used to gauge where these individuals are in the course of their illness, it is possible that the duration of viral shedding could be longer or shorter than 10 days after their first positive test.
-
Test-based strategy. Exclude from work until:
- Negative results of an FDA Emergency Use Authorized COVID-19 molecular assay for detection of SARS-CoV-2 RNA from at least two consecutive nasopharyngeal swab specimens collected ≥24 hours apart (total of two negative specimens). Note, because of the absence of symptoms, it is not possible to gauge where these individual are in the course of their illness. There have been reports of prolonged detection of RNA without direct correlation to viral culture.
Viral Detection of SARS-CoV-2 Over Time [5]
- Study of viral detection by RT-PCR (from throat and deep nasal cavity) at different timepoints of SARS-CoV-2 from 56 recovered COVID-19 patients not admitted to ICU from 3 hospitals in China
- If 2 consecutive negative results were achieved, the period between symptom onset and the date of 1stnegative RT-PCR test results was defined as viral nucleic acid conversion time
- RT-PCR Positive:
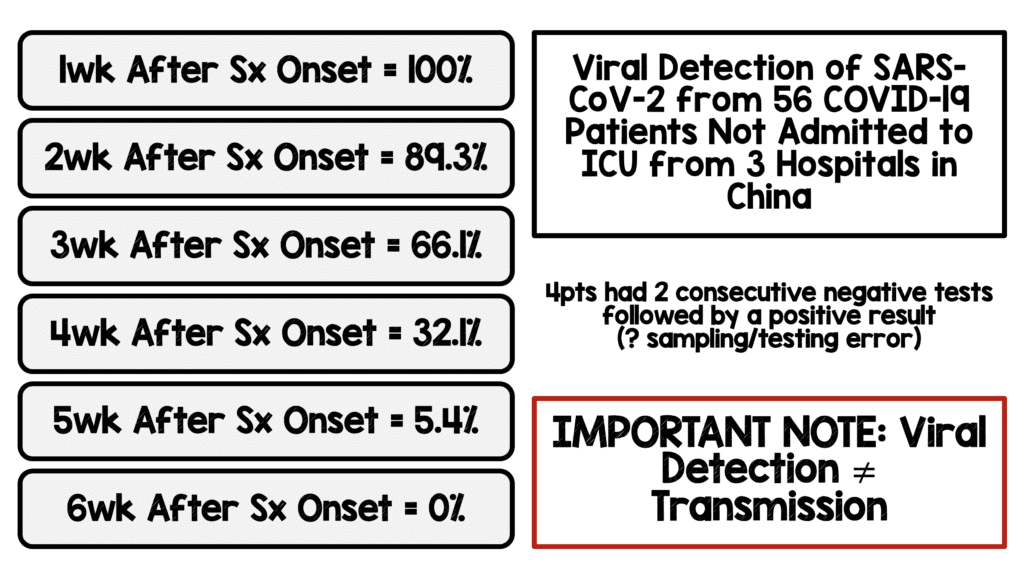
- Patients with >24 days of positive test were more likely to be older, have more comorbidities (i.e. DM, HTN)
- Errors in Sampling and Testing: 4 patients were found to have two consecutive negative RT-PCR tests followed by a positive result
- IMPORTANT NOTE: Viral Detection DOES NOT = Transmission
Serologic IgM and IgG Testing
- Analyzed the diagnostic performance of rapid IgM/IgG testing in COVID-19 pneumonia patients [6]
- Performed 3 analyses:
- 45 healthy control patients
- 55 confirmed by PCR patients
- 63 patients diagnosed with pneumonia of unknown etiology that were SARS-CoV-2 negative by PCR
- Healthy Controls: 45/45 had negative serologic test (Specificity 100%)
- Confirmed COVID-19: 26/55 (Sensitivity: 47.3%)
- Median time from onset of symptoms = 11d
- In patients with ≥14d from onset of symptoms = 17/23 (Sensitivity 73.9%)
- Pneumonia of Unknown Etiology:
- Median time from onset of symptoms 17d
- Positive 56/63 (88.9%)
- Positive in patients with ≥14d from onset of symptoms = 41/45 (91.1%)
- Bottom Line: Serologic testing is a nice complement to PCR to diagnose SARS-CoV-2 in patients with ≥14d from the onset of symptoms
- Performed 3 analyses:
- 208 plasma samples collected from 82 confirmed and 58 probable cases [7]
- The diagnostic value of serologic IgM testing was evaluated
- Median duration of IgM antibody detection was 5d after symptom onset
- Median duration of IgG antibody detection was 14d after symptom onset
- Overall Positive rate:
- IgM: 85.4%
- IgG: 77.9%
- Positive Rate in Confirmed Cases:
- IgM: 75.6%
- Positive Rate in Probable Cases:
- IgM: 93.1%
- Bottom Line: Positive detection rate increased (98.6%) when combined IgM ELISA assay and PCR tests for each patient when compared with a single PCR test (51.9%)
- The diagnostic value of serologic IgM testing was evaluated
Interpreting Diagnostic Tests for SARS-CoV-2 [8]
-
RT-PCR
- Most commonly used and reliable test for diagnosis
- Measured using a cycle threshold (Ct) = number of replication cycles required to produce a signal (i.e. lower Ct value represents higher viral RNA load)
- Viral RNA becomes detectable as early as day 1 of symptoms and peaks within the 1st week of symptom onset
- Positivity starts to decline by week 3 and subsequently becomes undetectable (In severely ill patients, PCR positivity may persist beyond 3 weeks after illness onset)
- A positive PCR only reflects detection of viral RNA and does not indicate presence of viable virus in all cases
- In some cases viral RNA has been detected up to 6 weeks [5] and a few cases have reported positive tests after 2 consecutive negative PCR tests performed within 24hrs (This why a “symptom based strategy” makes sense for workers to return to work if “at least 3days have passed since recovery defined as resolution of fever without the use of fever-reducing meds and improvement in respiratory symptoms + at least 10 days have passed since symptom onset)
- In a study of 205 patients [9] with confirmed COVID-19, RT-PCR positivity from highest to lowest was: BAL (93%), sputum (72%), nasal swab (63%), and pharyngeal swab (32%)
- Specificity of most of the RT-PCR tests is near 100%
-
Serologic Antibodies
- Indirect measurement of host immune response to SARS-CoV-2
- This test is more beneficial in the time window of 2 weeks after symptom onset
- By the 3rd – 4th week after symptom onset, is the peak window for IgM and IgG seroconversion
- By week 5 after symptom onset IgM begins to decline and almost disappears by week 7
- IgG however remains persistently elevated beyond 7 weeks
- ELISA-based IgM and IgG antibody tests have a greater than 95% specificity for the diagnosis of COVID-19
- The majority of antibodies that are produced are against the most abundant protein of the virus which is the nucelocapsid = Sensitivity
- The receptor-binding domain of the S protein is the host attachment protein = Specificity
- Therefore tests using both antigens for detection of IgG and IgM would result in a higher sensitivity and specificity
- Unfortunately antibodies can have cross-reactivity with SARS-CoV and other coronaviruses
- The long-term persistence and duration of protection conferred by neutralizing antibodies currently remains unknown
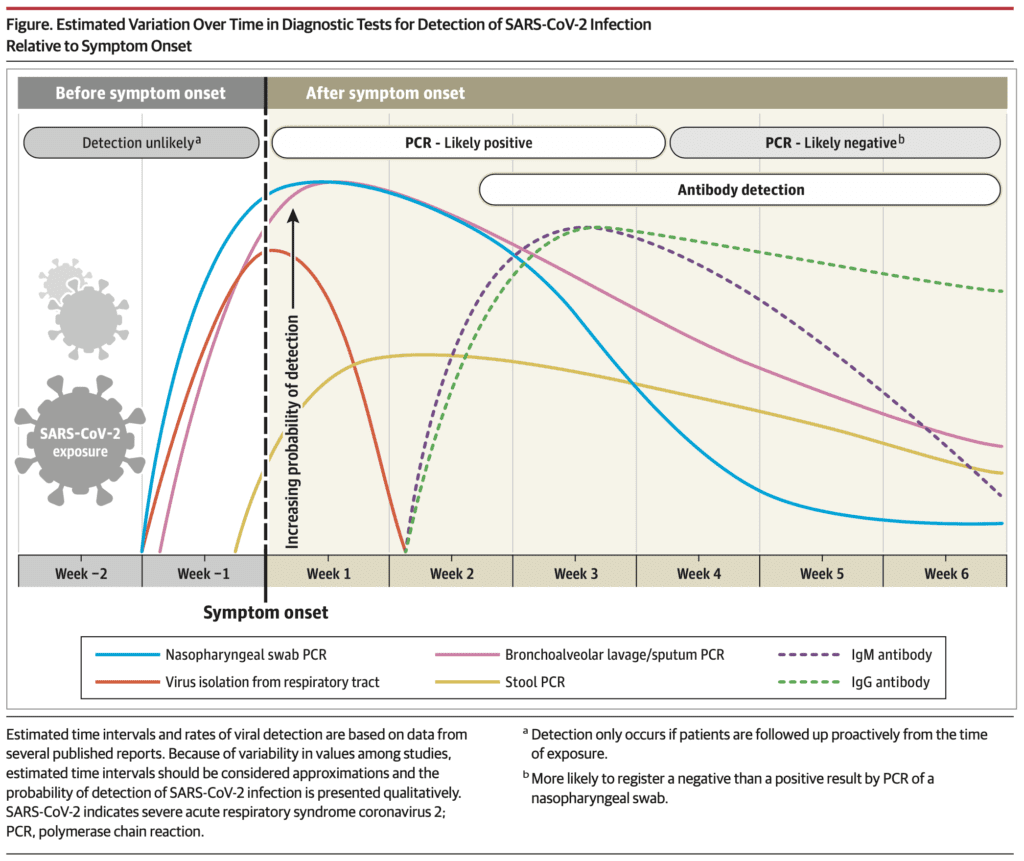
Proposed Timeline of Testing for SARS-CoV-2
References:
- Liu Y et al. Viral Dynamics in Mild and Severe Cases of COVID-19. Lancet Infectious Disease 2020. [Epub Ahead of Print]
- Arentz M et al. Characteristics and Outcomes of 21 Critically Ill Patients with COVID-19 in Washington State. JAMA 2020. [Epub Ahead of Print]
- Zhou F et al. Clinical Course and Risk Factors for Mortality of Adult Inpatients with COVID-19 in Wuhan, China: A Retrospective Cohort Study. Lancet 2020. PMID: 32171076
- Chen J et al. Findings of Acute Pulmonary Embolism in COVID-19 Patients. Lancet 2020. [Epub Ahead of Print]
- Xiao AT et al. Profile of RT-PCR for SARS-CoV-2: A Preliminary Study from 56 COVID-19 Patients. Clinical Infectious Diseases 2020. [Epub Ahead of Print]
- Perez-Garcia F et al. Rapid Diagnosis of SARS-CoV-2 Infection by Detecting IgG and IgM Antibodies with an Immonochromatographic Device: A Prospective Single-Center Study. medRxiv Pre-Print 2020. [Epub Ahead of Print]
- Guo L et al. Profiling Early Humoral Response to Diagnose Novel Coronavirus Diseases (COVID-19). Clin Infect Dis 2020. PMID: 32198501
- Sethuraman N et al. Interpreting Diagnostic Tests for SARS-CoV-2. JAMA 2020. [Epub Ahead of Print]
- Wang W et al. Detection of SARS-CoV-2 in Different Types of clinical Specimens. JAMA 2020. [Epub Ahead of Print]
For More Thoughts on This Topic Checkout:
- REBEL EM: COVID-19 – The Novel Coronavirus 2019
- CDC: Information for Healthcare Professionals
- EM Cases: Ep 137 – COVID-19 Part 1 – Screening, Diagnosis and Management
- EM Lit of Note: COVID-19 is EVERYWHERE
- FOAMCast: COVID-19 – Antibody Testing
Post Peer Reviewed By: Anand Swaminathan, MD (Twitter: @EMSwami) and Mizuho Morrison, DO (Twitter: mizuhomorrison)
The post COVID-19: Screening, Testing, PUI, and Returning to Work appeared first on REBEL EM - Emergency Medicine Blog.