
 Background: Currently, there are no approved medications for the treatment of COVID-19, but, there are many investigational agents that have shown antiviral activity against SARS-CoV-2 in vitro. Unfortunately in vitro studies do not always extrapolate to clinical care In vitro studies of remdesivir demonstrate inhibition of human and animal coronaviruses tested including SARS-CoV-2. However, the clinical and antiviral efficacy of remdesivir in COVID-19 remains to be established. The title of this post is, “two more trials just published on Remdesivir,” but in reality it is 1.5 trials as we don’t have the full release of the 2nd trial (see discussion).
Background: Currently, there are no approved medications for the treatment of COVID-19, but, there are many investigational agents that have shown antiviral activity against SARS-CoV-2 in vitro. Unfortunately in vitro studies do not always extrapolate to clinical care In vitro studies of remdesivir demonstrate inhibition of human and animal coronaviruses tested including SARS-CoV-2. However, the clinical and antiviral efficacy of remdesivir in COVID-19 remains to be established. The title of this post is, “two more trials just published on Remdesivir,” but in reality it is 1.5 trials as we don’t have the full release of the 2nd trial (see discussion).
Paper: Wang Y et al. Remdesivir in Adults with Severe COVID-19: A Randomised, Double-Blind, Placebo-Controlled, Multicentre Trial. Lancet 2020. [Epub Ahead of Print]
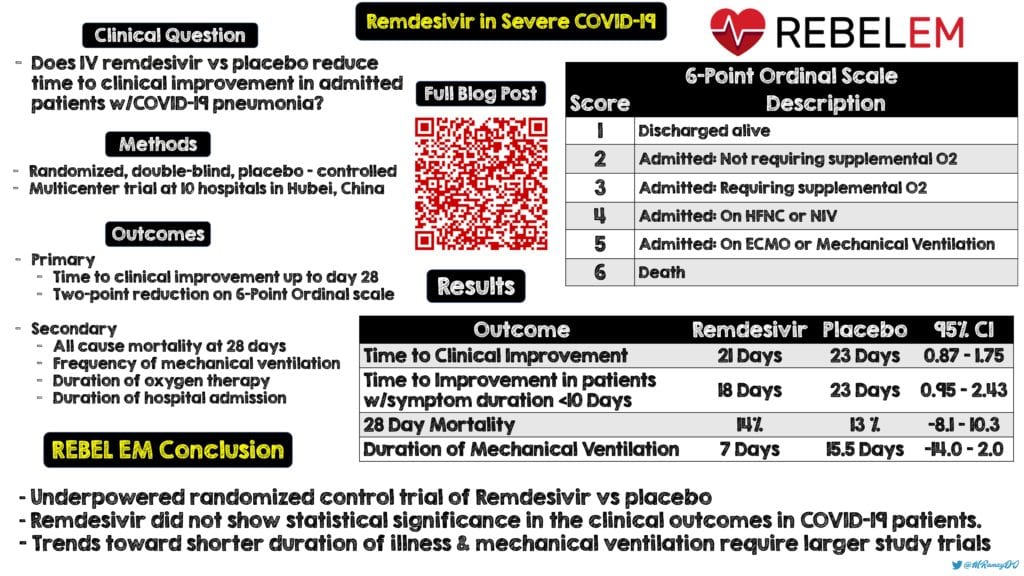
Clinical Question: Does IV remdesivir given over 10 days reduce the time to clinical improvement compared to placebo in patients admitted to the hospital with COVID-19 pneumonia?
What They Did:
- Randomized, double-blind, placebo-controlled multicenter trial at 10 hospitals in Hubei, China
- Patient randomized in 2:1 ratio to:
- IV remdesivir (200mg on day 1, followed by 100mg qd on days 2 – 10)
- Placebo x10d
- Patients allowed concomitant use of lopinavir-ritonavir, interferons, and corticosteroids
Outcomes:
-
Primary: Time to clinical improvement up to day 28 (Time in days from randomization to the point of decline of two levels on a six-point ordinal scale of clinical status or discharged alive from hospital, whichever came 1st)
- 6 Point Ordinal Scale
- 6 = death
- 5 = Hospital Admission for ECMO or Mechanical Ventilation
- 4 = Hospital admission for NIV or HFNC
- 3 = Hospital admission for O2 therapy (but not requiring HFNC or NIV)
- 2 = Hospital admission but not requiring O2 therapy
- 1 = Discharged or having reached discharge criteria (Defined as clinical recovery including normalization of pyrexia, RR <24BPM, O2 sat >94% on RA, relief of cough, all maintained for at least 72hrs)
-
Secondary:
- Proportion of patients in each category of the 6-point scale at day 7, 14, and 28 after randomization
- All-cause mortality at day 28
- Frequency of invasive mechanical ventilation
- Duration of oxygen therapy
- Duration of hospital admission
- Proportion of patients with nosocomial infection
-
Virologic Measures:
- Viral RNA detected
- Viral RNA load
-
Safety Outcomes:
- Treatment-emergent adverse events
- Serious adverse events
- Premature discontinuation of study drug
- 6 Point Ordinal Scale
Inclusion:
- ≥18 years of age
- Admitted to hospital with SARS-CoV-2 (confirmed by PCR test)
- Interval from symptom onset to enrollment of ≤12d
- O2 saturation of ≤94% on room air or P/F ratio ≤300mmHg
- Radiologically confirmed pneumonia
Exclusion:
- Pregnancy
- Breast feeding
- Hepatic cirrhosis
- ALT or AST >5x upper limit of normal
- Known severe renal impairment (eGFR <30mL/min/1.73m2)
- Receiving continuous RRT, HD, or peritoneal dialysis
- Possibility of transfer to a non-study hospital within 72h
- Enrollment into a n investigational treatment study for COVID-19 in the 30d prior to screening
Results:
- 237 patients enrolled
- Remdesivir: 158 patients
- Placebo: 79 patients (1 pt removed consent)
- Majority of patients were in category 3 (hospital admission requiring supplemental O2) of the ordinal scale (≈82% of enrolled patients)
- Median time from symptom onset to starting study treatment 10d (Range 9 to 12d)
- 66% of patients received corticosteroids during hospitalization with a median time from symptom onset to treatment of 8 days
- 39% of patients received corticosteroids prior to enrollment of study
-
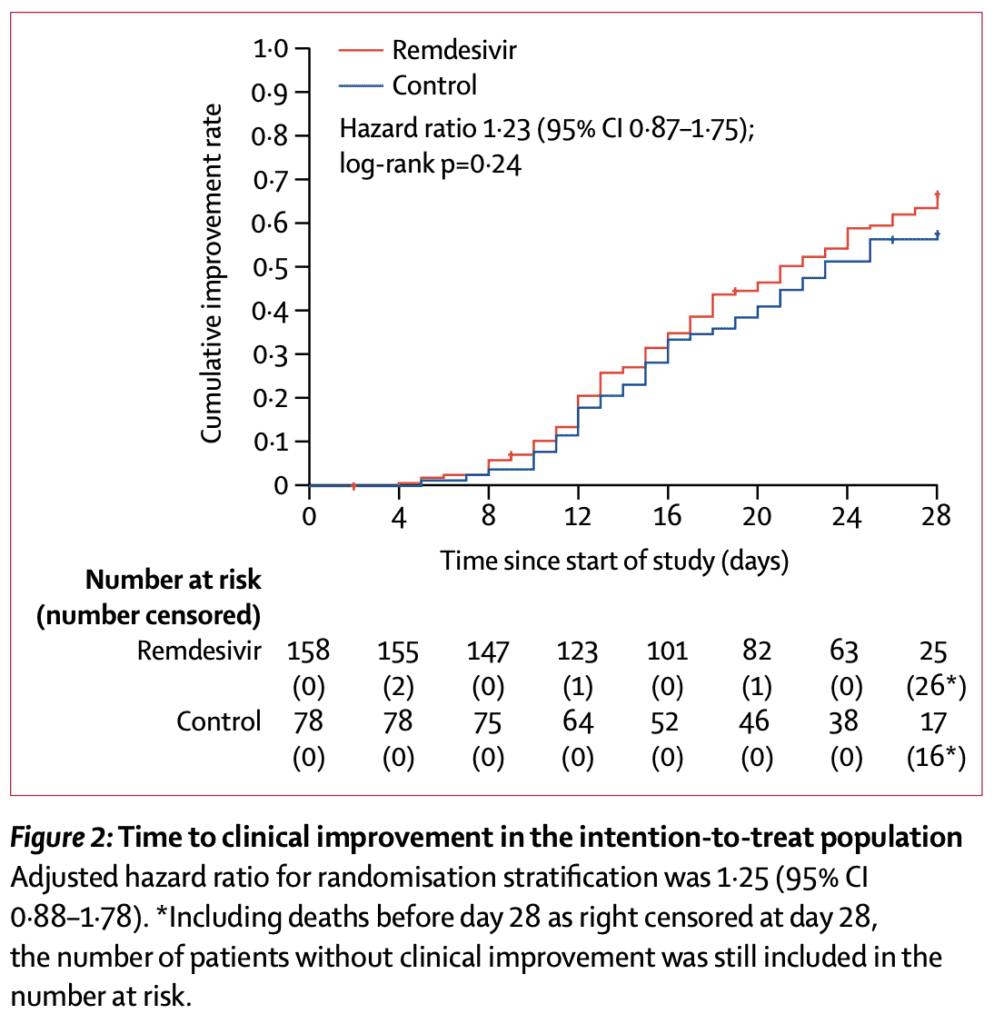
Median Time to Clinical Improvement (Primary Outcome):
- Remdesivir: 21d
- Placebo: 23d
- Not statistically significant (HR 1.23; 95% CI 0.87 to 1.75)
- 28d Mortality:
- Remdesivir: 14%
- Placebo: 13%
- 95% CI -8.1 to 10.3
- No difference in 28d mortality when remdesivir used within 10d after symptom onset (remdisivir 11% vs placebo 15%), however when used after 10d of symptom onset the 28d mortality rate was numerically higher (remdesivir 14% vs placebo 10%), but neither outcome was statistically significant
- Duration of Mechanical Ventilation:
- Remdesivir: 7.0d
- Placebo: 15.5d
- 95% CI -14.0 to 2.0 (Not statistically significant)
- Duration of invasive mechanical ventilation in survivors also numerically lower in remdesivir group (19d vs 42.0d) but again not statistically significant
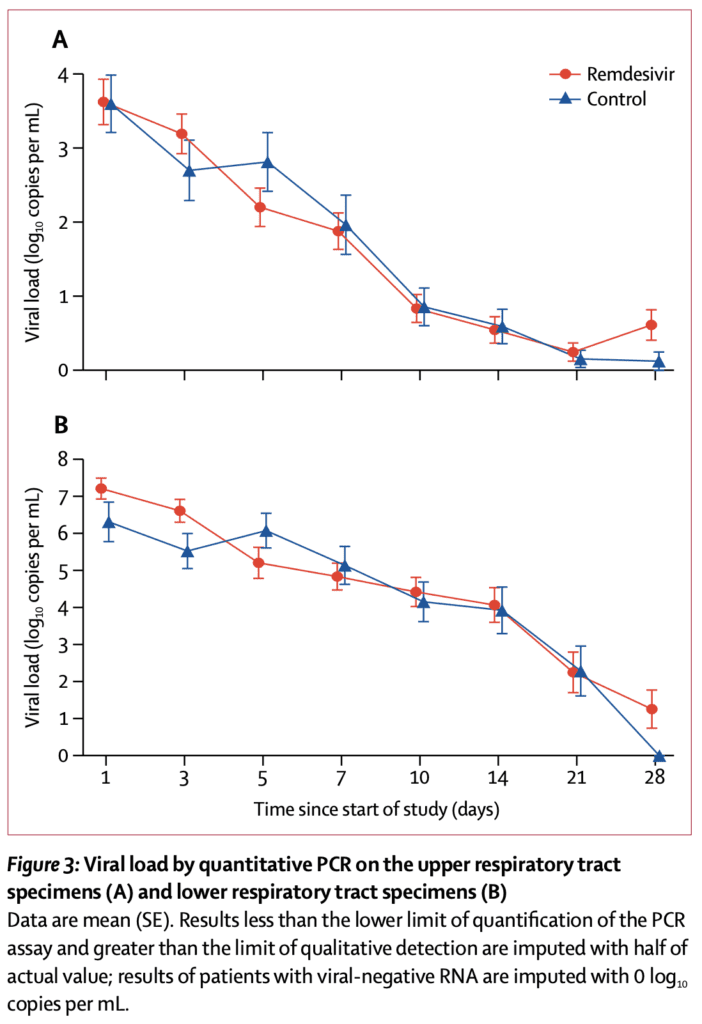
- Viral Outcomes:
- Remdesivir did not clear virus faster than placebo
- Adverse Events:
- Remdesivir: 66%
- Placebo: 64.0%
Strengths:
- Randomized, double-blind, placebo-controlled trial
- Randomization well performed
- Used an intention-to-treat analysis with all randomly assigned patients included which extrapolates better than a per protocol analysis to general practice
- Patients allowed concomitant use of lopinavir-ritonavir, interferons, and corticosteroids, however these were balanced between groups
- The number of adverse events were too small to make any concrete conclusions about safety
Limitations:
- Patients in the remdesivir group seemed to have slightly more comorbidities (HTN, DM, CAD), a respiratory rate >24, and the placebo group had been symptomatic for ≤10d more commonly. This makes me wonder if this group was slightly sicker than placebo and may have favored placebo at baseline
- Study was terminated early reducing the statistical power from 80% to 58% (i.e. fragile results). The reason for this were due to stringent public health measures used in Wuhan led to marked reductions in new patient presentations and restrictions on hospital bed availability
- Patients enrolled in this study were not very ill (i.e. 0.4% on invasive mechanical ventilation or ECMO)
- The frequent use of corticosteroids may have promoted viral replication of SARS-CoV-2, as was observed in SARS and MERS, but these studies only reported prolongation of detection of viral RNA, not infectious virus
- It is unclear whether higher doses or longer treatment courses of remdesivir would be beneficial in patients with severe COVID-19
Discussion:
- Most patients in this study had moderate illness, but not severe illness. Also many received medications relatively late, which is likely outside of the viral phase of illness. This could be one of the reasons we don’t see a decrease in viral loads.
- The National Institute of Allergy and Infectious Diseases (NIAID) published preliminary data from an RCT involving 1063 patients with COVID-19 on April 29th, 2020 [2]
-
Trial: Adaptive COVID-19 Treatment Trial (ACTT) was sponsored by the NIAID
- 68 sites (47 in the US and 21 in Europe/Asia)
- Patients randomized to remdesivir vs placebo
- Primary endpoint: Time to recovery (i.e. being well enough for hospital discharge or returning to normal activity)
-
Results:
- Recovery Time: Patients receiving remdesivir had a 31% faster time to recovery than placebo (P<0.001)
- Median Time to Recovery: 11d for remdesivir vs 15d for placebo
- Mortality Rate: 8.0% for remdesivir vs 11.6% for placebo (p = 0.059)
- We will have to wait for the full report, but this is the 1st report of a positive trial thus far (Looking at the clinicaltrials.gov website it looks like changes have already been made to their study in the middle of enrollment, which always makes me skeptical)
-
Trial: Adaptive COVID-19 Treatment Trial (ACTT) was sponsored by the NIAID
Changes Made to Primary Submission of ACTT Trial [Link is HERE]
- The pharmacokinetics of remdesivir in severely ill patients is unknown. Studies of higher-dose regimens for which there are safety data (i.e. 150 – 200mg/day) warrant consideration in future trials
- Although the overall proportion of patients with serious adverse events was lower in the remdesivir group compared to placebo it is important to remember more patients in the remdesivir group had dosing prematurely stopped by the investigators due to adverse events including GI symptoms, aminotransferase/bilirubin increases, and worsened cardiopulmonary status
- Although not statistically significant, remdesivir had a numerically faster time to clinical improvement vs placebo (18d vs 23d) in patients with symptom duration of 10d or less (HR 1.52; 95% CI 0.95 to 2.43). The authors play this up, but this was not a prespecified endpoint and it is unclear how clinical improvement was exactly defined.
Author Conclusion: “In this study of adult patients admitted to hospital for severe COVID-19, remdesivir was not associated with statistically significant clinical benefits. However, the numerical reduction in time to clinical improvement in those treated earlier requires confirmation in larger studies.”
Clinical Take Home Point: This underpowered, randomized clinical trial of remdesivir vs placebo did not show statistical significance in any of the clinical outcomes in patients with COVID-19. However, despite lack of statistical significance there were trends toward shorter duration of illness and duration of mechanical ventilation that require further study in larger trials.
Infographic Summary via Mark Ramzy, DO (Twitter: @MRamzyDO)
References:
- Wang Y et al. Remdesivir in Adults with Severe COVID-19: A Randomised, Double-Blind, Placebo-Controlled, Multicentre Trial. Lancet 2020. [Epub Ahead of Print]
- NIH Clinical Trial Shows Remdesivir Accelerates Recovery from Advanced COVID-19. NIAID 2020. [Epub Ahead of Print
For More on This Topic Checkout:
- PulmCrit: First Placebo-Controlled RCT on Remdesivir for COVID-19
- First10EM: Remdesivir – The First Real Trial
- The Bottom Line: Remdesivir for COVID-19 – A RCT
Post Peer Reviewed By: Anand Swaminathan, MD (Twitter: @EMSwami)
The post COVID-19: Two More Trials Just Published on Remdesivir appeared first on REBEL EM - Emergency Medicine Blog.


