
 Background: In end-stage renal disease (ESRD) patients on hemodialysis (HD), infection is the second most common cause of mortality after cardiovascular disease (Sarnik 2000). Because of the systemic inflammation and increased capillary permeability, septic patients are at significant risk for fluid imbalances and frequently require large volumes of crystalloids. The Surviving Sepsis Campaign guidelines provide a strong recommendation with low quality of evidence for administering a 30mL/kg fluid bolus within 3 hours of recognition of sepsis-induced hypoperfusion (Rhodes 2016). Further fluid administration should be guided by hemodynamic assessment (bedside echocardiography, passive leg-raise, etc.). In the general population, this early administration of fluids to patients with hypotension or sepsis-induced hypoperfusion has been associated with improved outcomes.
Background: In end-stage renal disease (ESRD) patients on hemodialysis (HD), infection is the second most common cause of mortality after cardiovascular disease (Sarnik 2000). Because of the systemic inflammation and increased capillary permeability, septic patients are at significant risk for fluid imbalances and frequently require large volumes of crystalloids. The Surviving Sepsis Campaign guidelines provide a strong recommendation with low quality of evidence for administering a 30mL/kg fluid bolus within 3 hours of recognition of sepsis-induced hypoperfusion (Rhodes 2016). Further fluid administration should be guided by hemodynamic assessment (bedside echocardiography, passive leg-raise, etc.). In the general population, this early administration of fluids to patients with hypotension or sepsis-induced hypoperfusion has been associated with improved outcomes.
However, there is significant confusion regarding the effects of a large 30mL/kg bolus on ESRD patients due to a lack of studies. While these patients may appear, volume overloaded on physical exam, they may be intravascularly volume deplete. Physicians may be hesitant to administer a large fluid bolus in ESRD patients because of the risk of precipitating cardiogenic shock, pulmonary edema, and respiratory failure. In fact, multiple studies show that patients who have ESRD are less likely to receive the full 30mL/kg fluid bolus compared to non-ESRD patients (Lowe 2018, Truong 2019, Dagher 2015). Furthermore, some studies show equivalent outcomes between ESRD patients who receive the full bolus and those who do not. We will review two studies that examined this topic.
Article #1: Rajdev K et al. Fluid Resuscitation in Patients with End-Stage Renal Disease on Hemodialysis Presenting with Severe Sepsis or Septic Shock: A Case Control Study. J Crit Care 2020. PMID: 31733623.
Clinical Question: Among patients diagnosed with severe sepsis and septic shock, what percentage of patients with ESRD on HD received an initial fluid resuscitation of ≥30mL/kg compared to non-ESRD patients?
What They Did:
- Retrospective case-control chart review study at a single center in Staten Island, New York
- Population
-
Inclusion Criteria
- Adult patients who met criteria for severe sepsis or septic shock based on SSC definitions admitted from 2015 to 2018
- Severe Sepsis = 2 SIRS criteria + sepsis-induced organ dysfunction or tissue hypoperfusion (SBP≤90mmHg or MAP≤70mmHg or fall of >40mmHg from baseline or lactate≥4mmol/L)
- Septic shock = sepsis-induced hypotension persisting despite a 30mL/kg fluid bolus
-
Exclusion Criteria
- Patients <18 years old
- Pregnant patients
- Patients with incomplete or illegible documentation
- Included patients were assigned to one of two groups
- Case (ESRD patients)
- Control (non-ESRD patients)
-
Inclusion Criteria
Outcomes:
- Primary: Percentage of patients who received initial fluid resuscitation of ≥30mL/kg presenting to the hospital with severe sepsis or septic shock
-
Secondary:
- Incidence of mechanical ventilation for respiratory distress
- Duration of mechanical ventilation
- Time to order antibiotics since presentation
- Hospital LOS and in-hospital mortality
- ICU admission and ICU LOS
- Incidence of urgent dialysis for volume overload
- Need for vasopressors and number of vasopressors required during hospitalization
- Source of infection
- The investigators also planned to perform a subgroup analysis of the same outcomes comparing the subset of ESRD patients who received ≥30mL/kg vs those who received <30mL/kg for severe sepsis and septic shock.
Results:
- 715 patient records were screened from 2015 to 2018
- 215 met criteria for severe sepsis and septic shock and were divided into two groups
- Case: 104 patients with ESRD on HD
- Control: 111 patients without ESRD
-
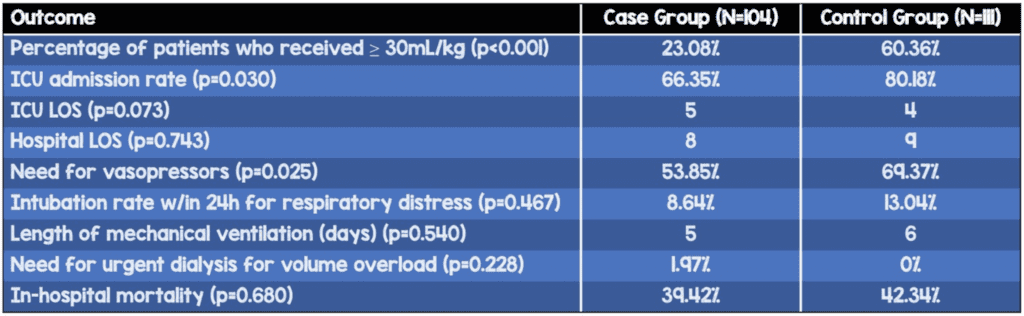
Percentage of patients who received ≥30mL/kg within the first 6 hours (p<0.001)
- Case: 23.08%
- Control: 60.36%
- Mean fluids received (p<0.001)
- Case: 21.38mL/kg (1643.3mL)
- Control: 36.28mL/kg (2669.8mL)
- ICU admission rate (p=0.03) – higher in control group
- Case: 66.35%
- Control: 80.18%
- Need for vasopressors (p=0.025) – higher in control group
- Case: 53.85%
- Control: 69.37%
- Rate of intubation for respiratory distress (within 24 hours of presentation), length of mechanical ventilation, mean time to ordering antibiotics, need for urgent dialysis for volume overload, and in-hospital mortality were not significantly different between groups
- 38 patients were excluded from the intubation rate calculation as they had a DNI order
- Source of infection
- Most common cause of severe sepsis or septic shock in both groups was pneumonia (Case: 45.19%, Control: 47.75%)
- UTIs were more common in the control group (27.93%) than in the case group (6.73%)
- Skin/Soft tissue/IV line infections were more common in the case group (19.23%) than in the control group (3.60%)
- Multivariate logistic regression analysis
- Age was significantly associated with mortality (p=0.044); for every one-year increase in age, the mortality increased by around 2.9%
- Higher lactic acid was associated with higher mortality (p<0.01)
- Fluid administration was independently associated with lower mortality in patients with severe sepsis and septic shock (p=0.035)
- Presence of ESRD was not found to be independently associated with mortality
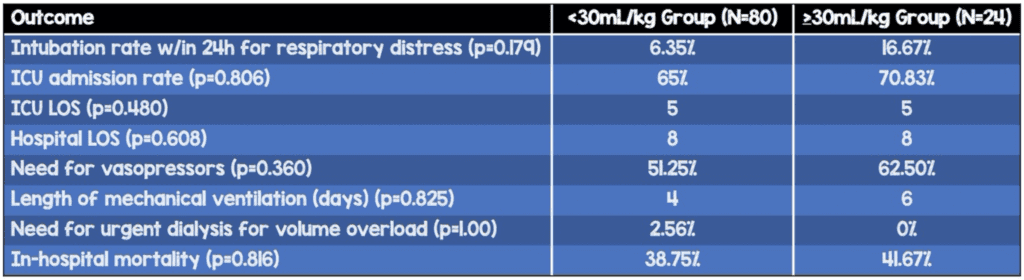
- Subgroup analysis: Compared ESRD patients who received <30mL/kg (80 patients) to those who received ≥30mL/kg (24 patients)
- No statistically significant difference in need for vasopressor support, number of vasopressors required, rate of ICU admission, ICU LOS, hospital LOS, rate of intubation, rate of urgent dialysis, and mortality. However, there was a trend towards worse outcomes with larger fluid boluses.
- Intubation rate within 24h of presentation for respiratory distress
- <30mL/kg: 6.35%
- ≥30mL/kg: 16.67%
- ICU admission
- <30mL/kg: 65%
- ≥30mL/kg: 70.83%
- Vasopressor use
- <30mL/kg: 51.25%
- ≥30mL/kg: 62.50%
- Length of mechanical ventilation (days)
- <30mL/kg: 4
- ≥30mL/kg: 6
- In-hospital mortality
- <30mL/kg: 38.75%
- ≥30mL/kg: 41.67%
Strengths:
- Compared to previous studies, the investigators had a relatively large sample size (215 total patients) included in the analysis.
- The investigators conducted a subgroup analysis focused on the outcomes of ESRD patients only. They asked whether aggressive (≥30mL/kg) vs restrictive (<30mL/kg) fluid resuscitation led to higher rates of intubation, urgent dialysis, hospital LOS, ICU LOS, need for vasopressors, in-hospital mortality, and other outcomes. In my opinion, this is the most revealing aspect of the study, but unfortunately it is a small sample size. It is important to understand that these were secondary outcomes and any results from this subgroup analysis are hypothesis-generating only.
- Many of the secondary outcomes are patient-oriented outcomes; however, they are secondary outcomes and therefore hypothesis-generating only.
- Except for a higher CCI, worse creatinine/GFR, lower heart rate, and mildly lower blood pressure parameters, the ESRD group was relatively similar to the control group.
Limitations:
- The single-center retrospective study design limits generalizability. Their sample was predominantly comprised of Caucasian patients, which was consistent with the population distribution of Staten Island, New York, but may not be applicable to other institutions. As a retrospective study, we do not know why providers chose to give more or less volume to which patients.
- The type of crystalloid and use of blood products or albumin was not recorded. The volumes and characteristics of these other products could have affected outcomes.
- The sample size in the subgroup analysis was small (total 104). A larger sample size may have revealed a difference in outcomes.
- The authors only looked at intubation within the first 24 hours. Some patients may have required intubation after this cutoff of one day.
- 38 patients were excluded from the intubation rate calculation as they had a DNI order.
- The baseline characteristics were largely the same with the following exceptions in the ESRD group:
- higher Charlson Comorbidity Index (CCI) – as expected in ESRD patients
- higher creatinine – as expected in ESRD patients
- lower GFR – as expected in ESRD patients
- lower DBP and MAP
- lower heart rates
- lower lactic acid
- The Non-ESRD group had higher lactic acid, heart rate, ICU admission rate, and need for vasopressors. This may indicate that the non-ESRD group was sicker at baseline, thus biasing the outcomes.
- Early administration of appropriate antibiotics has been shown to consistently reduce mortality. However, it is unclear what antibiotics were given in this study and whether they followed local antibiograms or other protocols.
- It is unclear how many of the ESRD patients were anuric or oliguric, which can also play an important role in deciding how much fluid to give, as well as outcomes.
- In the multivariate logistic regression analysis for mortality, they report that increasing age, lower volume of fluid administration, and higher lactic acid levels were associated with mortality. However, despite having confidence intervals not crossing 1.0, the odds ratios for these three items were all extremely close to 1.0, implying that the association with mortality was very mild. The odds ratios were:
- Age: 1.029
- Fluids: 0.981
- Lactic Acid: 1.15
- Age and lactic acid seem to be better predictors of outcome than ESRD itself.
- Their primary objective looked at percentage of ESRD patients with severe sepsis and septic shock who received ≥30mL/kg fluid bolus compared to non-ESRD patients. Not only has it already been demonstrated in the literature that a difference exists (Lowe 2018, Truong 2019, Dagher 2015), but also this is not a patient-oriented outcome. The patient-oriented outcomes in this study were all secondary outcomes; it is unclear whether this study was powered to adequately assess those.
- In the subgroup analysis comparing ESRD patients who received <30mL/kg vs ≥30mL/kg, the intubation rate is more than double in patients who received ≥30mL/kg. These patients also had higher ICU admission rate, longer length of mechanical ventilation, and higher vasopressor requirement. Despite not being statistically significant, this is not something we should completely ignore. It is entirely possible that with a larger sample size, this difference could potentially be statistically significant.
Author’s conclusion: “Our study indicates that initial fluid resuscitation of 30mL/kg may be safe, or at least not harmful, in patients with ESRD on HD and can potentially be incorporated in the sepsis triage bundle in the ED.”
Article #2: Khan RA et al. Association Between Volume of Fluid Resuscitation and Intubation in High-Risk Patients With Sepsis, Heart Failure, End-Stage Renal Disease, and Cirrhosis. Chest 2020. PMID: 31622591.
Clinical Question: Is fluid resuscitation with guideline-recommended volume (30mL/kg) associated with a greater risk of respiratory failure requiring intubation compared with those who received less than guideline-based volumes?
What They Did:
- Retrospective cohort study at a single center (Cleveland Clinic)
- Population
-
Inclusion Criteria
- Patients admitted to MICU between 2014 and 2016 with coding for sepsis and septic shock, who also had preadmission CHF, ESRD, or cirrhosis
-
Exclusion Criteria
- Transferred from outside hospitals
- Missing primary outcome data
- Sepsis episodes from a second or subsequent ICU admission
- Shock secondary to etiologies other than sepsis
- Patients intubated prior to initial fluid bolus
- Included patients were assigned to one of two cohorts:
- Fluid-restricted group (<30mL/kg crystalloids)
- Standard group (≥30mL/kg crystalloids)
-
Inclusion Criteria
Outcomes:
- Primary: Intubation within 72 hours following fluid bolus
-
Secondary:
- Time to intubation
- Change in oxygen requirement
- Alive ICU-free days
- Ventilator days
- Hospital mortality
- Propensity score matching analysis
- Multivariable logistic regression for intubation
Results:
- 2,517 eligible patients; 2231 excluded; 286 patients included; propensity score matching yielded 208 matched patients (104 matched pairs)
- Note: all of these patients had sepsis or septic shock AND CHF, ESRD, or cirrhosis.
- Fluid-restricted group N=104
- Standard group N=104
- Groups were evenly matched on many different factors
- 27% of patients in the study had ESRD
- 71% of patients in the study had CHF; 78% of these had EF of >40%
-
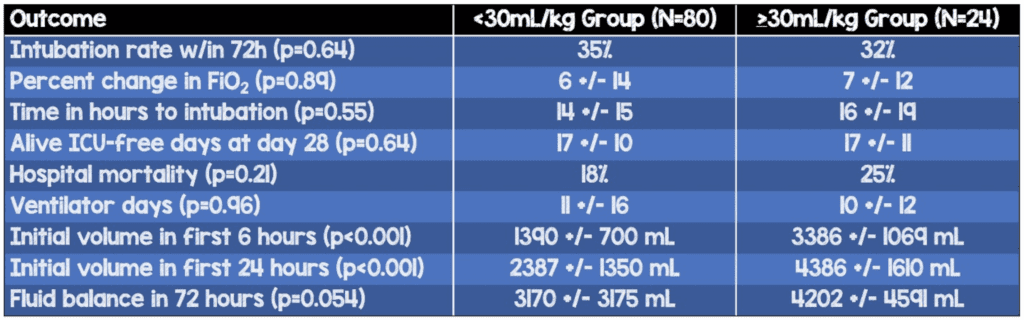
Percentage of patients who were intubated within the first 72h (p=0.64)
- Fluid-restricted: 35%
- Standard: 32%
- Following adjustment for several factors, administration of ≥30mL/kg was not independently associated with intubation (adjusted odds ratio 0.75; 95% CI, 0.41-1.36; P=0.34)
- Alive 28-day ICU-free days (p=0.64)
- Fluid-restricted: 17±10
- Standard: 17±11
- Ventilator days (0.96)
- Fluid-restricted: 11±16
- Standard: 10±12
- Hospital mortality (p=0.21)
- Fluid-restricted: 25%
- Standard: 18%
- Initial volume in first 6 hours (p<0.001)
- Fluid-restricted: 1390±700 mL
- Standard: 3386±1069 mL
- Initial volume in first 24 hours (p<0.001)
- Fluid-restricted: 2387±1350 mL
- Standard: 4386±1610 mL
- Fluid balance in 72 hours (p=0.054)
- Fluid-restricted: 3170±3175 mL
- Standard: 4202±4591 mL
Strengths:
- Rigorous identification process for sepsis and septic shock; they utilized a modified version of sepsis clinical surveillance definition by Rhee et al.
- Specifically looks at patients admitted to the ICU, and therefore, these patients were likely sicker. This study does not include patients who were admitted to the general floor.
- The propensity score matching created almost identical groups. The main exceptions were creatinine clearance at diagnosis, administered fluid amounts (as expected given the study design), and Do Not Intubate status (slightly higher in the standard group).
- They looked at intubation rates within 72 hours following fluid bolus, compared to shorter or longer time periods.
- Their outcomes were relevant, patient-oriented outcomes.
Limitations:
- Single-center retrospective study limits generalizability and cannot establish causality. The Cleveland Clinic is a well-established academic institution with access to advanced echocardiography, laboratory resources, and hemodynamic monitoring.
- This study also included patients with CHF and cirrhosis. Only about 27% of the study population had ESRD; we must be careful to not make any strong conclusions based on this study alone. Approximately 71% of all patients had heart failure, and 78% of these patients had an EF >40%. This may explain why the fluids were overall so well-tolerated.
- From a retrospective study, it is hard to know exactly why a patient did or did not receive a large fluid bolus. Perhaps, the clinicians gave ≥30mL/kg only to patients who they believed would tolerate the fluids. This could falsely skew the results in favor of the proposed conclusion.
- It is unknown why patients in the fluid-restricted group did not receive the recommended volume of fluid resuscitation; the authors speculate that it could have been due to their underlying comorbidity or that the patients clinically appeared to be less sick or had alternative plausible diagnoses.
- Unlike the Rajdev et al., the investigators of this study did not specifically look at intubation for respiratory distress. It is unclear whether they included all causes of intubation in their primary outcome.
- 302 patients were excluded due to missing data or repeat entries and we have no information on what happened to these patients. We also do not know what happened to the other 78 patients that were not propensity score matched.
- Again, it is unclear how many of these patients were oliguric or anuric and what the local antibiotic administration practices were.
- Fluid volume given by EMS is unknown and was not included in the study.
- Maintenance fluid volume was not recorded; only the fluid bolus volume given within the first 6 hours of sepsis diagnosis was recorded for this study.
- Only crystalloid was included in the volume calculation; blood products or colloids were not included.
- Excluded patients undergoing chronic dialysis in their reporting of creatinine clearance at diagnosis and AKI.
- Of note: There was no statistically significant difference in noninvasive ventilation use between the groups, but there was a significant difference in DNI status. The authors mention that 3 cases of NIPPV were used in DNI patients but do not specify which group. While it may be important to consider which group those patients belonged to, it may not affect the results significantly since it was only 3 patients.
Author’s conclusion: “We detected no difference in the incidence of intubation in the high-risk cohort of cirrhotic, heart failure, and ESRD patients with sepsis who received SSC guideline-concordant (30mL/kg) vs restricted fluid resuscitation.”
Discussions and Conclusions:
- Article #1 showed that ESRD patients with sepsis are more likely to receive less resuscitation fluids than patients without ESRD.
- Article #2 showed no difference in intubation rates within 72 hours, hospital mortality, ICU-free days, and ventilator days. The subgroup analysis in Article #1 also showed no significant difference in several outcomes including rate of intubation, hospital mortality, and urgent dialysis.
- Both studies address questions important to our practice in the emergency department every day. They provide good evidence that the 30mL/kg bolus of IV fluids for severe sepsis/septic shock is likely not causing increased harms in our ESRD patients, who are at risk for fluid overload.
- Requiring intubation is not an objective outcome. This is a subjective decision that varies among providers and can bias the outcomes.
- It has been shown that overall positive fluid balance during the entire ICU stay is associated with increased mortality. In these studies, the focus was on the initial fluid resuscitation rather than the total fluid balance during the ICU stay.
- These studies specifically targeted patients with severe sepsis and septic shock (ie. sicker patients). The evidence may not be applicable to patients who simply meet two SIRS criteria and have a suspected infection: sepsis.
- However, this is a good time to consider the million-dollar question of “Does this patient in front of me, need more fluids right now?” This is a complex question that requires a comprehensive evaluation of the patient’s current fluid status. Fluid responsiveness is a state where administration of fluid will lead to improvement in stroke volume and cardiac output. We all know that blood pressure, cool extremities, CVP, lactic acid, and other static measures are not good markers for fluid responsiveness. We need to determine which patients are still on the upward sloping section of the Frank-Starling Curve and selectively give these patients the fluids they need.
Utilizing a variety of static and dynamic indices, we can determine if a patient will be fluid responsive:- Trending capillary refill
- Echocardiography to determine stroke volume (SV = VTI x π x [LVOT diameter/2]2, or just eyeball it…) before and after passive leg raise of 250-500mL fluid challenge
- IVC ultrasound
- Pulse pressure variation (PPV)
- Stroke volume variation (SVV)
- End expiratory occlusion test (EEOT)
- There are a number of limitations to some of the measures of fluid responsiveness mentioned above. For example, PPV, SVV, and EEOT require intubation and mechanical ventilation and a normal, regular heart rate. IVC ultrasound can be erroneous if measured off-axis or in patients who generate large negative intrathoracic pressures (patients with asthma or emphysema). Patients with chronic pulmonary hypertension or cor pulmonale may have a chronically dilated IVC, but still be responsive to fluids. There is some newer research suggesting that PPV changes, in response to temporary increases in tidal volume (and even recruitment maneuvers), may be associated with fluid responsiveness.
- Moreover, it is challenging to measure dynamic indices of fluid responsiveness in a busy emergency department, where there is always another patient to be seen.
- I would recommend Haney Mallemat’s video about fluid responsiveness and several ways to determine whether the patient in front of you will be fluid responsiveness. “When you have a hemodynamically unstable patient, give that patient all the fluid they need, but not one drop more.” – Paul Marik
Clinical Take Home Point: In patients who have end-stage renal disease and severe sepsis or septic shock, we can give the 30mL/kg fluid bolus recommended by the SSC guidelines within the first 6 hours of resuscitation. Based on these two retrospective studies, administration of this fluid bolus does not seem to be associated with worse outcomes such as increased intubation, need for urgent dialysis, or hospital mortality. However, use careful assessments of your patient’s physiology to determine whether they require more fluids. Further prospective research is needed to determine whether fluid bolus administration causes harm or not.
Guest Post By:

For More On This Topic Check Out:
- emDocs: Sepsis with Comorbidities: Management Strategies
- Pulmcrit: Six myths promoted by the new surviving sepsis guidelines
- Pulmcrit: Myth-busting the fluid bolus
- The Short Coat: Fluid Resuscitation in Patients with Sepsis and Heart Failure, ESRD, or Cirrhosis
References:
- Sarnak MJ et al. Mortality caused by sepsis in patients with end-stage renal disease compared with the general population. Kidney Int 2000. PMID: 11012910.
- Rhodes A et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med 2017. PMID: 28101605.
- Lowe KM et al. Clinical Factors and Outcomes of Dialysis-Dependent End-Stage Renal Disease Patients with Emergency Department Septic Shock. J Emerg Med 2018. PMID: 29107479.
- Truong TN et al. Adherence to fluid resuscitation guidelines and outcomes in patients with septic shock: Reassessing the “one-size-fits-all” approach. J Crit Care 2019. PMID: 30784983.
- Abou Dagher G et al. Sepsis in hemodialysis patients. BMC Emerg Med 2015. PMID: 26467100.
- Rajdev K et al. Fluid Resuscitation in Patients with End-Stage Renal Disease on Hemodialysis Presenting with Severe Sepsis or Septic Shock: A Case Control Study. J Crit Care 2020. PMID: 31733623.
- Khan RA et al. Association Between Volume of Fluid Resuscitation and Intubation in High-Risk Patients With Sepsis, Heart Failure, End-Stage Renal Disease, and Cirrhosis. Chest 2020. PMID: 31622591.
- Rhee C et al. Incidence and Trends of Sepsis in US Hospitals Using Clinical vs Claims Data, 2009-2014. JAMA 2017. PMID: 28903154
Post Peer Reviewed By: Salim R. Rezaie, MD (Twitter: @srrezaie)
The post Fluid Administration in End-Stage Renal Disease for Severe Sepsis and Septic Shock by Shyam Murali, MD appeared first on REBEL EM - Emergency Medicine Blog.
