 Background: Despite the initial excitement around the use of chloroquine (CQ) and hydroxychloroquine (HCQ), there is mounting evidence that neither drug is effective in COVID-19 treatment. Laboratory studies have shown antiviral and immunomodulatory properties in vitro but these have not held up in clinical application. However, one potential area of use that needs more investigation is the use of HCQ for post-exposure prophylaxis (PEP). As the pandemic continues, PEP becomes an increasingly important topic in stopping repeat surges of the disease. To date, there is no high-quality evidence on prophylactic HCQ after exposure.
Background: Despite the initial excitement around the use of chloroquine (CQ) and hydroxychloroquine (HCQ), there is mounting evidence that neither drug is effective in COVID-19 treatment. Laboratory studies have shown antiviral and immunomodulatory properties in vitro but these have not held up in clinical application. However, one potential area of use that needs more investigation is the use of HCQ for post-exposure prophylaxis (PEP). As the pandemic continues, PEP becomes an increasingly important topic in stopping repeat surges of the disease. To date, there is no high-quality evidence on prophylactic HCQ after exposure.
Article: Boulware DR et al. A Randomized Trial of Hydroxychloroquine as Postexposure Prophylaxis for COVID-19. NEJM 2020. (Free Link)
Clinical Question: Does HCQ prevent symptomatic infection in patients exposed to SARS-CoV-2?
Population: Adults (> 18 years) who had household or occupational exposure to a person with confirmed COVID-19 at a distance of less than 6 ft for more than 10 minutes while wearing neither a face mask nor an eye shield (high-risk exposure) or while wearing a face mask but no eye shield (moderate-risk exposure). Recruitment was performed by social media outreach and traditional media platforms
Outcomes:
- Primary: Symptomatic illness confirmed by a positive molecular assay or, if testing unavailable, COVID-19 related symptoms
-
Secondary:
- Incidence of hospitalization for COVID-19 or death
- Incidence of PCR-confirmed SARS-CoV-2 infection
- Incidence of COVID-19 symptoms
- Incidence of discontinuation of the trial intervention
- Severity of symptoms at days 5 and 14
- Adverse events
Intervention: HCQ 800 mg X 1 followed by 600 mg 6-8 hours later then 600 mg Q24 X 4 days
Control: Matching placebo
Design: Randomized, double-blind, placebo-controlled trial in the US and parts of Canada
Excluded:
- Hospitalized
- HCQ allergy
- Retinal eye disease
- G6PD deficiency
- Chronic kidney disease (stage 4 or 5 or receiving dialysis)
- Porphyria
- Weight < 40 kg
- Receiving chemotherapy
- Concurrent use of HCQ, Azithromycin or cardiac arrhythmia medications
- Canadian additional exclusions: pregnancy, breastfeeding, severe diarrhea or vomiting, known cirrhosis with encephalopathy or ascites, known prolonged QT, history of ventricular arrhythmia or sudden cardiac death or QT prolonging medications.
- On 4/20/20, FDA issued additional exclusions: ischemic heart disease, personal or family history of prolonged QT, QT prolonging medications or weight < 50 kg
Primary Results:
-
-
Recruited 821 asymptomatic patients that were randomly assigned
- HCQ n = 414
- Placebo n = 407
-
Health care workers (HCW) n = 545 (66.4%)
- 342/545 (62.8%) were physicians and physician assistants
- High-risk exposures: 87.6% (719/821)
- Moderate-risk exposures: 12.4% (102/821)
- 60% of participants reported not wearing any PPE during their exposure
-
Recruited 821 asymptomatic patients that were randomly assigned
Critical Findings:
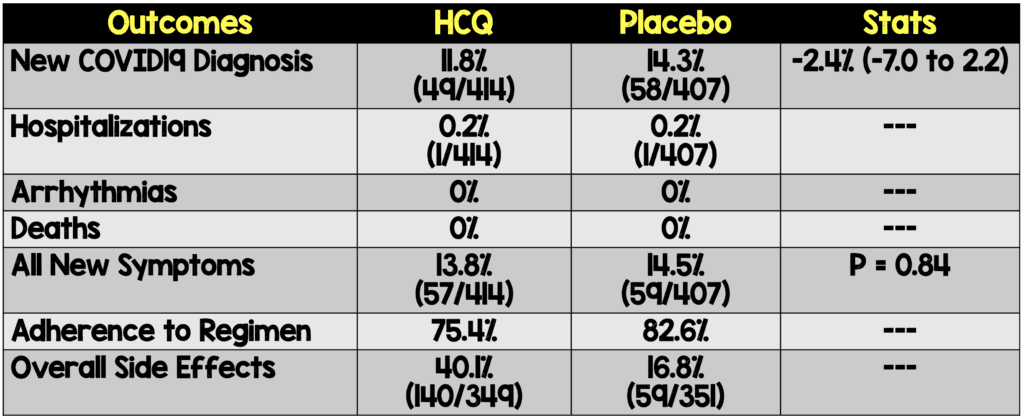
- Overall new COVID19 Diagnosis: 13%
- No serious intervention-related adverse reactions or cardiac arrhythmias
Strengths:
- Asks a clinically, epidemiologically and public health relevant question
- First published study with an RCT design looking at prophylaxis
- Groups were well balanced at baseline
- Pragmatic approach to diagnosis given the limited availability of COVID-19 testing in the US
- Randomization occurred at research pharmacies allowing blinding to be maintained
- Masking of interventions (placebo or HCQ) was good – participants did not show a reliable ability to identify what treatment arm they were enrolled in
- Loss to follow up was low: 11.7% of patients did not follow up at 14 days. Authors used multiple approaches to obtain follow up.
- Dosing regimen of HCQ was based on pharmacokinetic simulations to achieve plasma concentrations above the SARS-CoV-2 in vitro half maximal effective concentration for 14d
- 4 ID docs unaware of trial group reviewed symptomatic patients to generate a consensus with respect to whether condition met case definition of COVID-19
Limitations:
- Authors planned the study to look for a 50% relative reduction in cases which may have been overly ambitious. A smaller reduction would have required a much larger study
- Recruitment was primarily through media and this may have led to a bias in enrolled patients
- Exposure history (< 6 ft for > 10 minutes) is subjective and susceptible to recall bias
- Most patients were unable to be formally tested for SARS-CoV-2 due to lack of testing availability – it’s unclear if/how this affects the results
- Data regarding symptoms and SARS-CoV-2 test positivity were by patient report allowing for recall bias
Discussion:
- The authors performed an intention-to-treat analysis which reflects real world conditions but, per-protocol data would be useful to look at as well to see if a difference exists when the regimen is closely followed
- Initially, the group specified a second primary outcome: ordinal scale of COVID19 disease severity that was changed to overall change in disease severity over 14 days among those who are symptomatic at baseline. This data does not appear in the manuscript
- The authors note that there were no serious adverse reactions or arrhythmias, but, QT prolongation may be present without the researchers knowing (study participants didn’t all get ECGs) and, the study sample is too small to make robust safety comments
- Patients in this trial were generally younger and healthier than those at risk for severe COVID-19. May have seen different results in an older cohort with more comorbid disease
Authors Conclusions: “After high-risk or moderate-risk exposure to Covid-19, hydroxychloroquine did not prevent illness compatible with Covid-19 or confirmed infection when used as postexposure prophylaxis within 4 days after exposure.”
Our Conclusions: In this high-quality RCT, there is no evidence that HCQ is effective as post-exposure prophylaxis in reducing the development of symptomatic disease. With an absence of clinical equipoise, it is unclear if additional studies are warranted.
For More Thought on This Topic Checkout:
- The Bottom Line: Boulware
- St. Emlyn’s: Hydroxychloroquine for Prophylaxis in COVID19
Post Peer Reviewed By: Salim R. Rezaie, MD (Twitter: @srrezaie)
The post Hydroxychloroquine is Ineffective for Post-Exposure Prophylaxis appeared first on REBEL EM - Emergency Medicine Blog.
